How to Cite | Publication History | PlumX Article Matrix
Rajendra Jangde1*, Sanjay Daharwal1, Ram Kumar Sahu2 and Anurag Rajvanshi3
¹University Institute of Pharmacy, Pt. Ravishankar Shukla University, Raipur - 492 010 India.
²Oriental College of Pharmacy, Raisen Road, Bhopal - 462 021 India.
³Manav Bharti University, Laddo, Solan - 173 229 India.
Correspoding Author e-mail: rjangdepy@gmail.com
ABSTRACT: The purpose of study was to evaluate the potential of microspheres for taste masking when incorporated into orally disintegrating tablets. The tablets were produced by simple wet granulation technique with a model compound (Ampicillin and Cloxacillin) which is moderately bitter. The formulating procedure had two variables to obtain good taste masking with desirable characteristics. It was found that ethyl cellulose was the best for masking the unpleasant taste of ampicillin and cloxacillin among the six kind of polymers investigated. The influences of other formulation factor, i.e. dichloromethane-acetone ratios and ethyl cellulose polymer ratios on the properties of microspheres were also examined. In conclusion, the results of the present study will be helpful for the preparation of oral forms of Ampicilin and Cloxacillin with an acceptable taste.
KEYWORDS: Ampicilin; Cloxacillin; Microspheres; Taste masking
Download this article as:| Copy the following to cite this article: Jangde R, Daharwal S, Sahu R. K, Rajvanshi A. Formulation and Characterization of Tasteless Dispersible Tablet Containing Ampicilin and Cloxacillin. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Jangde R, Daharwal S, Sahu R. K, Rajvanshi A. Formulation and Characterization of Tasteless Dispersible Tablet Containing Ampicilin and Cloxacillin. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9647 |
Introduction
Although tablets and capsules constitute a major portion of the drug delivery systems, some patient groups, such as pediatrics, geriatrics, and bedridden or disabled patients, may have difficulties in swallowing such dosage forms. Many pharmaceutical manufacturers are now switching to orally disintegrating tablet (ODT) technology and offering a wider choice of pharmaceutical actives covering many therapeutic categories to both physicians and patients1. To meet these medical needs, formulators have devoted considerable efforts to develop a novel dosage form known as orally disintegrating tablet (ODT), which can disintegrate rapidly in the saliva without water However, taste masking for some pharmaceutical actives with bitter or unpleasant taste can be challenging for this dosage form to achieve patient acceptability. Elderly individual having difficulty in swallowing, found difficult in taking medications prescribed in conventional tablet and capsules form. The problem of swallowing is also evident in paediatrics patients. The dispersible tablets overcome all the above problems and thus offer an alternate form of oral medication, which provide patients with a more convenient means of taking their medication. The aim of the present study is to develop dispersible tablet formulation adopting a conventional process and compression equipment so that the technology implantation is easier and cost effective. A combination of super disintegrating agents will be added in the formulation to achieved rapid disintegration of the tablet in the oral cavity. An antibiotic drugs, ampicillin and cloxacillin are chose for the study, which is ideal candidate for dispersible tablet2-4.
Ampicillin and Cloxacillin, is beta-lactam antibiotic an effective in the treatment of urinary tract infection, respiratory tract infection meningits, gonrrhoea, typhoid fever etc.
Taste masking for some pharmaceutical actives with bitter or unpleasant taste can be challenging for this dosages form to achieve patient acceptability5.
The mechanism of the taste masking method may be summarized as following. The taste masks the distasteful sensation by addition of flavors, sweeteners and effervescent agents. The second is avoiding the bitter drug coming into direct contact with patients taste buds by coating6. Hence the purpose of study was to evaluate the potential of microspheres for taste masking when incorporated into orally disintegrating tablets.
Material and Methods
Materials
Ampicillin and Cloxacillin (Zim Labs. Pvt. Ltd., Nagpur) was used as a water insoluble model drug with a very bitter taste. Ethyl cellulose (Loba chemie. Pvt. Ltd., Mumbai) dicholoromethane (Loba chemie. Pvt. Ltd., Mumbai) acetone (Lobachemie. Pvt. Ltd., Mumbai), polyvinyl alcohal (Lobachemie. Pvt. Ltd., Mumbai) were used. All other chemicals and solvents used were of analytical reagent grade.
Preparation of microspheres
All microspheres were obtained by the emulsion solvent diffusion method using distilled water as an external phase, in which 1% of PVA was dissolved as an emulsifier. The internal phase consisted of a good solvent and a bridging liquid involving ampicillin, polymer7.
At first, the drug and polymer were co-dissolved in an organic solvent mixture that was composed of ethanol, acetone (good solvent) and dicholomethane (bridging liquid). The drug solution was slowly injected via a syringe into the external water phase (poor solvent) under agitating. The system was stirred continuously for about 1 hr. Along with the good solvent diffusing into the poor solvent, droplets gradually solidified and formed microspheres8,9.
Then, the system was filtered to separate the microspheres from the preparation system. The resultant product was washed with distilled water and dried in an oven at 400C for 12 hr. the whole process was carried out at room temperature10.
Taste evaluation
The taste study was checked by using panel method. For this, ten volunteers were selected. To each of the volunteers, solution of pure drug and suspension of microspheres in distilled water were given, and asked to put it on the tounge for 10 seconds to check the taste. Bitterness levels were recorded immediately and threafter 10 minutes on a 0-3 scale with 0 being thresold, 1 slight, 2 moderate and 3 strong bitter11,12.
Formulation study
Dispersible tablet containing 60 mg equivalent of Ampicilin and Cloxacillin were compressed on single stroke tablet machine (Rolex Ltd. Ambala). The dispersible base was used as a disintegrating agent. The granules of dispersible base were made by wet granulation method by mixing ethyl cellulose, maize starch, and aerosil in the ratio 10:7:313-15.
Tablet evaluation
The prepared tablets were evaluated for appearance, hardness, friability, and disintegration time, uniformity of dispersion, weight variation test and content uniformity test, which are as fallows16.
Appearance
Five tablets were broken and the broken sections were examined.
Hardness
Five tabletes were individually tested Monsanto hardness tester17, 18.
Friability (% loss)
Ten tablets accurately weighed were subjected to 4 minute friability testing using Roche friabillator. The tablets were collected. Weighed and the weight loss due to the friability was calculated as percentage of initial weight19, 20.
Disintegration time
Six tablets were tested in accordance to defined IP test (without disc) for dispersible tablet. This utilizes water at temperature of 19-210C21.
Uniformity of dipersion
Two tablets were placed in 100 ml of water at 19-210C and allowed dipersing. Dispersed suspension was subjected to pass through sieve no.22.
Weight variation test
Twenty tablets were weighed and the average weight was calculated. Each tablet was then weighed individually and compared with average weight23, 24.
Content uniformity test
Twenty tablets were crushed and the quantity equivalent to 60mg of the drug was weighed. The drug was analyzed after suitable treatment and dilution spectrophotometrically25, 26.
Dissolution studies
A dissolution test was performed at 37 °C using the USP type-1 basket apparatus at 25 rpm with 1000 ml 0.01N HCl as a dissolution medium. At predetermined intervals (5, 10, 15, 30 min), 5 ml of the medium was sampled and filtered and 5 ml of fresh dissolution medium was replaced. Analyzed after suitable dilution with by systronic 2101 UV-visible spectrophotometer at 520 and 480 nm. The same process was repeated for marketed control release product 250 mg27- 29.
Results and Discussion
Ampicilin and cloxacillin microspheres were prepared under the emulsion solvent diffuson method microsphere particles with moisture content less than 4% and drug release 98.56% cumulative percentage release dissolution rate of the active will determine the taste masking potential of these microspheres30,31. Particle size, morphology and intergrity of the particles have direct impact on the dissolution rate32. Dispersible tablets prepared from the powder of Ampicilin and Cloxacillin microspheres were uniform in appearance33. The tablets passed the test for weight uniformity (not more than 2 tablets differ from average weight by more than ± 5% and no tablet differs by more than ± 10%). Hardness was found to be 2.5 kg/cm2 and friability was less than 1% (Table 3). The tablet disintegrated in 100 seconds34,35. This was comparable with the release pattern of SR marketed tablets (Table 2, Figure 1)36. From the table 1 it was found that all the formulation release drug under prescribed time37 and it is illustrated in figure 2.The release pattern showed that the drug was instantaneously released in the stomach to provide a sustained effect upto 6 hours38,39.
Table 1: Dissolution profile of formulations.
| Sampling time (mins) | Cumulative % drug release | |||
| F1 | F2 | F3 | F4 | |
| 5 | 96.95 | 97.37 | 100.68 | 99.06 |
| 10 | 97.43 | 97.91 | 101.18 | 99.55 |
| 15 | 97.66 | 98.45 | 101.68 | 100.04 |
| 30 | 98.20 | 99.00 | 102.18 | 100.54 |
Table 2: Dissolution profile marketed formulation and optimized OD tablet.
| Sampling
time |
% of drug release | % of drug release
(F4 OD tablet) |
| 5 | 76.05 | 99.06 |
| 15 | 86.87 | 99.55 |
| 30 | 90.29 | 100.04 |
| 45 | 92.3 | 100.54 |
Table 3: Evaluation of formulated tablet.
| S.No. | Methods | Observations |
| 1 | Appearance | Uniform |
| 2 | Hardness( kg/cm2) | 2.5 |
| 3 | Friability (%loss) | 0.1 |
| 4 | Disintegration Time | 10.0 |
| 5 | Weight Variation | Complies |
| 6 | Content Uniformity | Good |
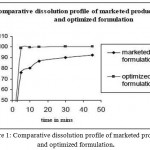 |
Figure 1: Comparative dissolution profile of marketed product and optimized formulation.
|
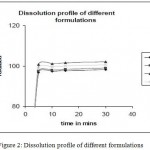 |
Figure 2: Dissolution profile of different formulations.
|
Acknowledgements
The authors wish to acknowledge the University institute of pharmacy, Pt. Ravi Shankar Shukla University, Raipur (C.G.) for providing equipments and facilities needed for this work.
References
- Alvarez-Fuente J., Fernandez-Arevalo M., Holgado M. A., Caraballo I., LLera J. M. and Rabasco A. M., Pharmazie, 49, 834–839 (1994).
- Barreto D. G., Curr. Therap. Res., 57, 79–86 (1996).
- Duchene D., Wouessidjewe D., and Ponchel, G., J. Contr. Release, 62, 263–268 (1999).
- Ferrara A., Dos Santos C., Cimbro M. and Gialdroni Grassi G., Int. J. Antimicrob. Agents, 7, 181–186 (1996).
- Fu X. Y. and Yang W. D., J. Hainan Med. Coll., 6, 4–6 (2000).
- Gao Y, Wang Y. and Zhang L., Int. J. Pharmaceutics, 318, 62-69 (2006).
- Saini T. R., Kaushik D. and Dureja H., The Indian Pharmacist, 3(19), 72-75 (2004).
- Daharwal S. J., Saraf S., Jha A. K. and Bhusari K. P., The Indian Pharmacist, 3(19), 79-84 (2004).
- Reddy K. R., Mutalik S. and Reddy S., AAPS Pharm. Sci. Tech., 4, E61 (2003).
- Assimopoulou A. N. and Papageorgiou V. P., J. Microencapsul., 21, 161-173 (2004).
- Ubrich N., Bouillot P., Pellerin C., Hoffman M. and Maincent P., J. Control. Release 97, 291-300 (2004).
- Assimopoulou A. N., Papageorgiou V. P. and Kiparissides C., J. Microencapsul. 20, 581-96 (2003).
- Agrawal A. M., Howard M. A. and Neau S. H., Pharm. Dev. Technol., 9, 197-217 (2004).
- Basit A. W., Podczeck F., Newton J. M., Waddington W. A., Ell P. J. and Lacey L. F., Eur. J. Pharm. Sci., 21,179-189 (2004).
- Shi X. Y. and Tan T. W., Biomaterials, 23, 4469-73 (2002).
- Al-Omran M. F., Al-Suwayeh S. A., El- Helw A. M. and Saleh S. I., J. Microencapsul., 19, 45-52 (2002).
- Rowe R. C., Sheskey P. J. and Weller P. J., Handbook of pharmaceutical excipients” American Pharmaceutical Association, Washington, 186-190 (2003).
- Fong J. W., CRC Press, Boca Raton, 81-100 (1998).
- Lucinda-Silva R. M. and Evangelista R. C., Acta Farm. Bonaerense 24, 366-370 (2005).
- Lucinda-Silva R. M. and Evangelista R. C., J. Microencapsul., 20,145-152 (2003).
- Cunliffe D., Kirby A. and Alexander C., Adv. Drug Deliv. Rev., 57, 1836-1853 (2005).
- Suedee R., Bodhibukkana C., Tangthong N., Amnuaikit C., Kaewnopparat S. and Srichana T., J. Control. Rel., 129,170-178 (2008).
- Cormack P. A. G. and Elorza A. Z., J. Chromatog., 804(1), 173-182 (2004).
- Spivak D. A., Adv. Drug Deliv. Rev., 57, 1779-1794 (2005).
- Ali M., Horikawa S., Venkatesh S., Saha J., Wook Hong J. and Byrne M. E., J. Control. Rel., 124, 154-162 (2007).
- Sajeev C., Vinay G., Archna R. and Saha R. N., J. Microencapsul., 19, 753-760 (2002).
- Chowdary K. P. R. and Madhvi B. L. R., Pharm. Rev., 11, 137-140 (2006).
- Karasulu H. Y., Taneri F., Sanal E., Guèneri T. and Ertan G., J. Microencapsul., 19, 357-362 (2002).
- Dandagi P. M., Manvi F. V., Gadad A. P., Mastiholimath V. S. and Patil M. B., Indian J. Pharm. Sci., 66, 631-635 (2004).
- Ghulam M., Ahmad M., Akhtar N., and Rasool F., Pak. J. Pharm. Sci., 22(3), 291-300 (2009).
- Duchene D., Wouessidjewe D. and Ponchel G., J. Contr. Release, 62, 263–268 (1999).
- Alvarez-Fuente J., Fernandez-Arevalo M., Holgado M. A., Caraballo I., LLera J. M. and Rabasco A. M., Pharmazie, 49, 834–839 (1994).
- Barreto D. G., Curr. Therap. Res., 57, 79–86 (1996).
- Duchene D., Wouessidjewe D. and Ponchel G., J. Contr. Release, 62, 263–268 (1999).
- Ferrara A., Dos Santos C., Cimbro M. and Gialdroni G.G., Int. J. Antimicrob. Agents 7, 181–186(1996).
- Fu X.Y. and Yang W.D., J. Hainan Med. Coll. 6, 4–6(2000).
- Gao Y., Wang Y. and Zhang L., Int. J. Pharmaceutics, 318, 62-69(2006).
- Saini T.R., Kaushik D., and Dureja H., The Indian Pharmacist, 3(19), 72-75(2004).
- Daharwal S.J., Saraf S., Jha A.K. and Bhusari K.P., The Indian Pharmacist, 3(19), 79-84(2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.





