How to Cite | Publication History | PlumX Article Matrix
F. A. Mahboub, A. M. Ali and F. A. Khorshid*
Department of Medical Biology, KAAU Head of Tissue Culture Unit , KFMRC, Saudi Arabia.
ABSTRACT: Green tea has shown promise in the prevention of several cancers due to its chemoprevention properties. This study investigated the role of green tea extracts against ovarian cancer metastasis of leukemic cells in female mouse model. Animals were divided to: control group, infected by leukemic cells and experimental group, which was infected then treated with green tea extracts on day (0) of infection. Animals were scarified after 2-10 weeks following injection; the ovaries were quickly removed then preceded for histological examinations by light and electron microscopy. Light microscopy results were well illustrated with their cyto-structure by using transmission electron microscopy. Histopathological examination of infected non-treated group revealed that, completely no follicles were shown, the entire ovary crowded with cancerous cells and L1210 cells attacked the ovarian parenchyma whereas, in treated group, significant improvement in histological and ultrastructural examination were observed in ovarian parenchyma and all follicles types reappeared again.
KEYWORDS: Green Tea Extracts; L1210 cells; Ovarian cancer; Histopathological; Ultrastructural
Download this article as:| Copy the following to cite this article: Mahboub F. A, Ali A. M, Khorshid F. A. Histopathological and Ultrastructural Study on the Effect of Green Tea Extracts on Ovarian Cancer Model. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Mahboub F. A, Ali A. M, Khorshid F. A. Histopathological and Ultrastructural Study on the Effect of Green Tea Extracts on Ovarian Cancer Model. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9305/ |
Introduction
Green Tea
Tea, especially green tea has shown promise in the prevention of several cancers [1]. A reduction in the risk for the development was observed for all subtypes of adult leukemia associated with the habitual high intake of GTEs [2]-[4] , reduction in the risk of breast cancer[5]-[7], prostate cancer [8] ,colorectal cancer [9]-[11], oral cancer [12], lung cancer[13], gastric cancer [14], endometrial endometroid adenocarcinoma [15], and prostate cancer [7]. Epidemiologic evidence is strongest for organs of the gastro- intestinal tract, possibly because of their direct contact with tea constituents [16]. [17], interestingly, the majority of studies reporting protective effects were conducted in Asian countries where GTEs is predominantly consumed.
In studies conducted in rodents, tea has exhibited strong anticarcinogenic activity against a number of carcinogens of human relevance including heterocyclic amines and polycyclic aromatic hydrocarbons that are important food contaminants, being formed during the normal cooking process [18].
The potent chemopreventive activity of GTEs has been observed on a number of target organs and has been attributed to the fact that it influences favorably all stages of carcinogenesis process, namely initiation, promotion, and progression as well as metastasis [19],[20]. GTEs can modulate the initiation stage by preventing the bending of genotoxic
case–control study was conducted in Hangzhou, China. The risk of epithelial ovarian cancer significantly declined with increasing consumption level and years of GTEs drinking. The risk of serious cell ovarian cancer appeared to have an even stronger inverse association with tea consumption. The follow-up study of the same patients reported that green tea affected the survival rate of ovarian cancer patients post diagnosis [28].Five other case–control studies [31]-[35] and one cohort study [36], also showed association between tea consumption and reduced risk of ovarian cancer.
There is ample evidence from in vivo studied indicating that components of GTEs are associated with decreased risk or progression of several cancers, relatively few studies have specifically investigated ovarian cancer. This stimulated the present investigation to study the effect of GTEs on induced ovarian cancer female mice model.
Material and Methods
Materials
Green tea extracts (GTEs)
Green tea was locally purchased from Green tea center Jeddah-KSA and stored at 4C° in sealed bag ,( 2.5% w/v)were prepared by adding boiling water (100 ml) to the tea (2.5 gm ) in flask ,leaving to stand for 10 -15 min., inverting every 30s ,and then filtering through cotton wool, according to [37].
Cell lines
Lymphocyte leukemia, mouse Cell lines L1210 – were obtained by cell strain from American type Culture Collection (ATCC) (Rockvill, Merland), available in the cell bank of the Tissue Culture Unit (TCU), King Fahd Medical Research Center (KFMRC) ,King Abdul-Aziz university (KAU) , Jeddah.
L1210 cells were maintained in PRMI (1640) [31] culture medium (10% FCS) before being inoculated into Balb/c mice at dose of 0.2 ml of leukemia cells (1×105 /ml of media) according to [38].
Animals
Forty health adult virgin female MFI strain mice were used, weighted 25-35g aged 5-6 weeks. They were purchased from the Animal House in King Fahd Medical Research center (KFMRC), (KAU), Jeddah, Kingdom of Saudi Arabia , kept in temperature of 25± 2 C° a relatively humidity of 40± 4 , 12/12 h light/dark cycle and free access drinking water and food . Each five animals were housed in plastic cages covered with galvanized metal and allowed to acclimate 7 days prior to the experiment. All animals received human care according to ethical requirements. Approved by, the Animals Research Ethic Committee of KAU. Animals were divided into two groups each group consist of twenty animals:
Group (1):- considered as infected group by L1210 cells.
Group (2):- considered as treated group with GTEs on (0) day of infection by L1210 cells.
Methods
All animal groups in groups (1) and (2) were i. p. injected by L1210 cells at dose level of (0.2 ml) according to [38].
Mice in group (1) were scarified after ascetic fluid in pelvic region was appeared due to leukemic infiltrated in most organs according to [26]. While mice in group (2) were scarified after injected with L1210 cells and administrated with GTEs for ten weeks on day (0) of infection.
All animals were sacrificed 2-10 weeks following injection; the ovaries (left and right) were quickly removed and used for histological work by light microscopy and ultrastructural work by transmission electron microscopy (TEM).
Results
Infected animals with L1210 Cells
Light Microscopy (LM) study
Histopathological examination of infected group which was injected only with L1210 cells revealed that, completely no follicles was shown; the entire ovary crowded with cancerous cells and L1210 cells attacked the ovarian parenchyma from outside (Fig.1a).
This evidence involvement by a malignant neoplastic lymphoid infiltration, exhibiting a massive and predominant diffuse pattern (Fig.1b).More subtle histological detail was apparent in semi-thin sections, found inside the ovary which surrounded by flattened germinal epithelium attached to L1210 cells from outside the ovary and rich in dilated blood vessels, the nuclear size and configuration were seen to be quite variable and the effected tumor cells could be easily identified with large cell has bizarre nuclei and high nuclear cytoplasmic ratio with scanty cytoplasm (Fig.1c)
The stroma contained frequent mitotic figures, cells with pyknotic nuclei and larger number of multinucleated cells were observed with indent nuclear membranes (Fig.1 d).
In routine paraffin – embedded Heamatoxlin and eosin-stained sections, tumor cells nuclei were round or ovoid, large and dark. Esinophilic macronuclei were rarely conspicuous. Infected ovary was highly vascular with large number of dilated congested blood vessel (Figs.1e&f).
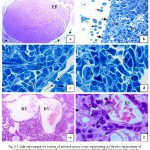 |
Figure 1: Light micrograph for section of infected mouse ovary representing.
|
Massive replacement of ovary parenchyma with L1210 cells, one vacuolated primary follicle (PF) and L1210 cells outside the ovary (arrows) (H&E x4). b) Round L1210 cells outside the ovary (arrows), infected germinal epithelium (dashed-arrows) and dilated blood vessels (BV) (TB x20). c) L1210 cells outside the ovary attached to flattened germinal epithelium (arrows). Tumor cells (TC) with large dark nuclei (TB x100). d) Tumor cells in mitosis (M) and bizarre multinucleated nuclei (arrows) (TB x100). e) Large number of dilated congested blood vessel (BV) (H&E x20). f) Infiltrated L1210 cells with prominent macronucleoli (arrows), metaphase (dashed-arrow) and dilated blood vessel (BV) (H&E x100).
Transmission Electron Microscopy (TEM) study
All the obtained light microscopy results were well illustrated with their cyto-structure by using transmission electron microscopy.
Ultrastructural study exhibited, ovarian germinal epithelium with squamous cancer cell have hypertrophied nucleus with high ratio of nucleoplasm followed by necrotic primary follicle in stroma. The stroma have bizzard multinucleated cancer cells and on the other hand, normal primordial follicle with large pale oocyte have mitotic nucleus with condensed chromosomes dispersed inside its cytoplasm adjacent to two follicular cells (Fig.2 a). With higher magnification necrotic primary follicle contain oocyte with convoluted nucleus have dispersed euchromatin, highly abundant condensed heterochromatin and prominent nucleolus surrounded by irregular, ruptured nuclear membrane Vacuolated mitochondria with no cristea were observed (Fig.2 b).
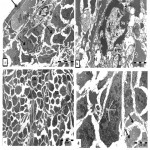 |
Figure 2: Transmission electron micrograph for section of external part of infected ovary with L1210 cells showing: a) .
|
Squamous cancer germinal epithelium cell (arrow) with hypertrophied nucleus, ubnormal primordial follicle (P) with large pale oocyte (O), nucleus in mitosis (M), two follicular cells (white arrows), necrotic primary follicle (PF) in stroma(S). Note, cells with convoluted nuclei (dashed-arrows) .b) Details necrotic primary follicle contain large oocyte (O), convoluted nucleus (N), dispersed euchromatin (EC), abundant heterochromatin(C) and marginal nucleolus (Nu), irregular and ruffled nuclear membrane (arrows). Vacuolated mitochondria (M) in limited cytoplasm c) Stroma (S) filled with disorganized cells varies in shapes, inbetween nemours mitotic cells (Mi). Fibrous layer (arrows). d) Elongated tumor cell with numerous nuclei (N) filled with chromatin masses. Note degenerated stroma cells (arrows).
Thin fibrous layer was noted surrounding tumor cells divide the stroma into compartments (Fig.2c). Large elongated tumor cell with numerous nuclei filled with chromatin masses and less amount of euchromatin surrounded by rare dense cytoplasm and poorly endowed with cytoplasmic organelle were seen .Also, there were degenerated stroma cells with pale cytoplasm have vacuolated organelles (Fig.2d). The stroma filled with disorganized cells varies in shapes inbetween numerous mitotic cells , poly nucleated tumor cells, invaded macrophage , neuron like structure, lytic cells, dilated blood vessel with dense endothelial cells and red blood corpuscles (Fig.3a).
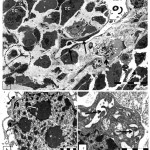 |
Figure 3: Transmission electron micrograph for section of internal part of infected ovary with L1210 cells showing :a).
|
Poly nucleated tumor cells (TC) , blood vessel (BV) with endothelial cells (arrows) contain red blood corpuscles ( RBC), lytic cells(arrow -head ). Neuron like structure (dashed -circle), infiltrated bizzard macrophage (dashed-arrow) and cells in mitosis (Mi). b) Detail of large macrophage nucleus (N) with multi nucleolei and blabbing nuclear membrane (dashed-arrow) and pinocytotic vesicle (arrow). c) Detail of secondary lysosome (arrows) in irregular scanty cytoplasm with undulating surface folds.
Invaded macrophage contain large nucleus have numerous nucleoli and irregularly dilated perinuclear cisterns forming blabbing (Fig.3 b).Macrophage cells with pinocytotic vesicles, numerous secondary lysosomes, and irregular scanty cytoplasm with undulating surface folds was seen (Fig.3 c).
Treated group with GTEs on day (0) of infection
Light Microscopy (LM) study
Histopathological study of the ovaries treated with GTES on day (0) after infection with L1210 cells for ten weeks in contrast to infected group revealed improvement in ovarian tissue, where nearly normal ovary with germinal epithelium, ovary parenchyma , all follicles types (primordial follicle, primary follicles, secondary follicle, atretic follicle and corpus luteum ) reappeared again. The germinal epithelium took the normal cuboidal shaped with central nucleus, some round secondary follicle with two or three layers of cuboidal granulosa cells with thin theca folliculi were observed , loose granulosa cells with large intercellular spaces contact with oocyte have pale nucleus surrounded by clear zona pellucida (Fig. 4a). Other secondary follicle with organized granulosa cells were detected in many regions in stroma with thin theca folliculi, clear zona pellucida, oocyte and nucleus (Fig.4b) In semi-thin sections good details appeared where some primordial follicle, secondary follicle with preapoptotic follicular cells and detached oocyte were recorded in stroma with large granular cells ((Fig. 4c).Apoptotic cells were evident by the dark blue stained and shrinkage nucleus, clumping of chromatin integrated with the nuclear membrane and loose of cell-cell contact (Fig.4 d). Graafian follicle with centric oocyte, antrum, granulosa cells, and corona radiata and theca folliculi were clearly observed (Fig.4e) and corpus luteum surrounded by theca folliculi and filled with preapoptotic cells was detected (Fig.4f).
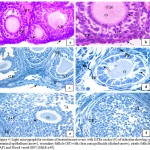 |
Figure 4: Light micrograph for sections of treated mouse ovary with GTEs on day (0) of infection showing: a).
|
germinal epithelium (arrow), secondary follicle (SF) with clear zona pellucida (dashed-arrow), atretic follicle (AF) and blood vessel (BV) (H&E x40). b) Cuboidal germinal epithelium (dashed-arrow), secondary follicle with thin theca folliculi (TF), granulosa cells (GC) ,clear zona pellucida( arrow), oocyte (O) and nucleus (N)( H&E X40). c) Germinal epithelium (arrow), primordial follicles (P), secondary follicle (SF) with granulosa cells (GC) and Graafian follicle (GF), (TB X20). d) Primary follicle (PF) with detached oocyte (O), preapoptotic cells have dilated intercellular spaces (arrows) and thin theca folliculi (TF) in stroma with large granular cells (dashed-arrow) (TB X100). e) Graafian follicle with centric oocyte (O), antrum (A), granulose cells (GC) , corona radiata(arrow)and theca folliculi (TF) (TB x40). f) Corpus luteum (CL) surrounded by theca folliculi (TF) and filled with preapoptotic cells (TB x40).
Transmission Electron Microscopy (TEM) studies
Normal squamous germinal epithelium cell with flat nucleus and primordial oocyte adjacent to follicular cells were recorded. Primordial oocyte have nucleus with clear nucleolus and numerous round and tubular mitochondria, part of theca folliculi and granulosa cells (Fig.5 a).
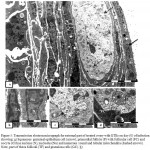 |
Figure 5: Transmission electron micrograph for external part of treated ovary with GTEs on day (0) of infection showing: a).
|
Squamous germinal epithelium cell (arrow), primordial follicle (P) with follicular cell (FC) and oocyte (O) has nucleus (N), nucleolus(Nu) and numerous round and tubular mitochondria (dashed arrows) . Note, part of theca folliculi (TF) and granulosa cells (GC). b) Details elongated germinal epithelium nucleus (N) with normal nucleolus (Nu) and dense nucleus of tunica albugenia (TA). c) Details of clear oocyte nucleus (N), peripheral heterochromatin (C), nucleolus (Nu) and well developed nuclear envelope (arrow) in oocyte cytoplasm. d) Details oocyte has round mitochondria (M) and follicular cell (FC) with lipid droplets (L).
Details of germinal epithelium showed that, it had elongated nucleus with normal nucleolus nearby tunica albugenia cell have dense nucleus (Fig.5b). Oocyte with clear nucleus, peripheral heterochromatin, nucleolus and nuclear envelop were clearly detected (Fig, 5 c). With higher magnification round pale mitochondria and lipid droplet surrounded by smooth membrane were seen in oocyte cytoplasm (Fig.5d). Healthy-looking primary follicle showed oocyte surrounded completely by one layer of large cuboidal granulosa cells and enclosed by cellular theca interna and fibrous theca externa inside the stroma (Fig.6a). Follicular cells were characterized with numerous round mitochondria, large nucleus and desmosomes connecting the cell together and oocyte inside the primary follicle revealed clear nucleus with dispersed finely granular chromatin, nucleolus, intact nuclear envelope and clustered mitochondria with pale matrix and peripheral cristae clouds in the oocyte cytoplasm (Fig.6 b).
The secondary follicle is surrounded by theca layers which differentiated into theca interna and theca externa and contained multiple layers of granulosa cells non-cellular zona pellucida around the oocyte separated the oocyte from the granulosa cells (Fig.6 c ).
The granulosa cells and oocyte represented cell projections and the granulosa cells connected together with desmosomes (Fig.6 d).
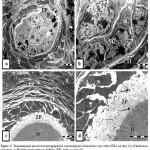 |
Figure 6: Transmission electron micrograph for external part of treated ovary with GTEs on day (0) of infection showing: a).
|
Healthy look primary follicle (PF) with oocyte (O) surrounded by one layer of cuboidal granulosa cells (GC) ,theca interna (arrow) and theca externa (dashed-arrow) in stroma cells (S). .b) Details oocyte (O) with indented nucleus (N) has nucleolus (Nu), granulosa cells (GC) with numerous round mitochondria (M) and desmosomes (white-arrow). c) Secondary follicle surrounded by theca interna (TI), and theca externa (TE) two layers of granulosa cells (GC), non cellular zona pellucida (ZP) surround large oocyte (O) in stroma (S). .d) Details of oocyte and granulosa cells projections ((arrows), granulosa cells (GC) with lipid droplets (L), nucleus (N). Note, desmosomes (white-arrow) connected granulosa cells together.
Discussion
Leukemic infiltration of the ovaries
Han et al. [26] reported that, leukemic infiltration of the ovaries is extremely rare, but ovaries can be the apparent site of involvement of the abdominal or pelvic viscera with leukemia .This explained our results in animals infected with L1210 cell (G2) infiltration and ended with ovarian cancer model. Histological examination for ovarian cancer model revealed small, round to oval cells with little cytoplasm and hyperchromatic nuclei lacking distinguishing features. Intermingled with these were occasional spindle cells and rhabdomyoblasts characterized by a round to oval configuration, relatively abundant, brightly eosinophilic cytoplasm, and small eccentric nuclei. Focally, nests of tumor cells separated by connective tissue trabeculae having a characteristic alveolar pattern. In other areas, the tumor was composed of pleomorphic cells, including elongate, strap-like, racket-shaped, or bizarre configurations. These cells had brightly eosinophilic cytoplasm, and single or, more often, multiple bizarre nuclei. Longitudinal fibrils and clumped fibrillary material in many of the tumor cells were revealed .The present observations were supported by [39].
The protective effect of green tea
Lin [40] reported that, the accumulation of reactive oxygen species (ROS) is the hallmark of oxidative stress has long been associated with intracellular events leading to protein, DNA and lipid damage. These oxidative stress-related intracellular events have also been shown to be strongly correlated to the increased incidence of diseases such as cancer. Reactive oxygen species (ROS) and mitotic signal activation are considered to be major factors in tumor development .ROS affect key transcription factors that are active mitotic signal transducers, leading to stimulation of cell proliferation, inflammation and tumor promotion. In terms of cancer chemoprevention, tea polyphenoles can inhibit several of these transcription factors, such as activator protein-1 and nuclear factor-κB, thereby blocking mitotic signaling pathways.
The mechanisms by which green tea may exert a protective effect on cancer are not yet fully understood. Ahmad, et al [41] studied epigallocatechin- 3-gallate (EGCG) which is the major component of the green tea polyphenols and is primarily responsible for the green tea effect. It is important to note that (EGCG) resulted in the induction of apoptosis in all carcinoma cells but not in the normal cells .This results supported our findings, where apoptosis detected in the treated group and its recognition is commonly based on morphological criteria as cell shrinkage, blabbing, specific chromatin condensation and loss of cell-cell contacts [42] and this led to the reappearance of the different follicles in the treated group.
The demonstration of induction of apoptosis by epigallocatechin-3-gallate is also important because green tea is a well consumed beverage worldwide and has shown promising cancer chemopreventive effects in both laboratory experiments and human epidemiologic studies. A vast variety of the chemotherapeutic agents, currently used in cancer therapy, are thought to kill the cells by the mechanisms other than apoptosis. This may not always be a preferable way of cancer management [41].Apoptosis plays a crucial role in eliminating the mutated hyper proliferating cells from the system [43] .Thus, induction of apoptosis in tumor cells may be considered as a protective mechanism against development and progression of cancer [41],[44].
The ultrastructural features of apoptosis are relatively easily recognized. A complicating factor is relatively low frequency in which apoptotic cells are observed, especially in lieu of the rapid phagocytosis of these cells in vivo [45]-[47]. Associated with the nuclear condensation, the biochemical hallmark of apoptosis is an extensive cleavage of the nuclear DNA into oligonucleosome-sized fragments. These fragments can be visualized as the typical ladder pattern using gel electrophoresis and [48] DNA breaks can be detected in situ using enzymatic labeling system [49].
While epigallocatechin- 3-gallate (EGCG) is thought to be the most important component of green tea, it is likely that tea polyphenoles may work synergistically so that green tea may have stronger anticancer effects than EGCG or any other component alone [50]. However, recent research reported a protective effect of green tea on ovarian cancer risk [51] which appeared in our experiment where the preventive effect of green tea well recorded.
It is hypothesized that the antioxidant effect of green tea is responsible for its chemoprevention and disease-modifying properties [3], (several interventional studies have demonstrated that habitual green tea consumption causes an increase in plasma antioxidant status and quantitative reduction of free radical-induced markers of lipid peroxidation.
Halder et al. [52] indicated that, both theaflavins (TF) and thearubigins (TR) polyphenoles induce apoptosis via mitochondria-mediated pathway and involvement of proapoptotic and antiapoptotic proteins, thus providing a molecular basis for understanding the chemopreventive effect of TF and TR that might be ideal candidates to induce effective apoptosis in cancer cells.
Animal studies have demonstrated that both EGCG and theaflavins in tea can induce apoptosis [53] and cell-cycle arrest in many cancer cell lines [19]. It appears that green tea can selectively induce apoptosis in cancer cells and not normal cells [19]. A molecular mechanism proposed for these effects involves activation of the p53 protein and induction of the expression of Bax and p21, primary responser activation genes for p53, all of which are involved in accelerating programmed
cell death[25],[54]. These findings imply that green tea has the potential for cancer prevention by inhibiting tumor development and in cancer progression by preventing tumor invasion, proliferation and angiogenesis [55].
Green tea’s cancer fighting benefits may be due to a component of the drink that prevents angiogenesis, the process of blood vessels growth .Tumors are depended on the continual development of new blood vessels to grow and multiply, it was believed that EGCG may be active in preventing the growth of new blood vessels in tumors, and could be the green tea’s association with lower cancer incidence [56]. In our study with laboratory mice, we found less blood vessel growth in mice that were given green tea than in cancer ovarian model.
One of feature which was noted in many sections of ovaries of animals treated with GTEs and cannot described was the presence of numerous large granular cells randomly scattered throughout the ovary. The granules on low power inspection under the microscope, the first impression given was that they consisted of deposits of stain. They could be compared with the tissue mast cells of the mouse, such as those found in the dermis. They were equivalent to the latter in size, gave metachromatic reaction with Toluidine blue [57].
Conclusion
However, the antiproliferative role of Green Tea has been delineated in case ovarian cancer only. These observations will add new revolution in the field of developing therapeutic strategies for cancer in near future. To our knowledge, this is the first report of the induction of apoptosis by green tea in ovarian cancer.
Acknoledgment
Authors’ gratefully acknowledge King Abdul-Aziz City for Science and Technology Saudi Arabia for their financial support, Prof. G. Y. Dehlawi Professor of Reproductive Physiology, Histological Slid Preparation unit especially Mrs. Khloud Al-braiky for her excellent technical assistance and Electron Microscopy Unit in King Fahd Medical Research Center in King Abdul-Aziz University.
Rrefrences
- A.H. Lee, M.L. Fraser and C.W. Binns, “Possible role for …..greinto Balb/c mice at dose of 0.2 ml of leukemia cells (1×105 /ml of media) according to [38].
- en tea in ovarian cancer prevention” Future Oncol., …. …..vol.1, no. 6, and pp.771-777.2005
- Lin, S. Kikuchi and A. Tamakoshi, et al., “Green tea …..consumption and the risk of pancreatic cancer in Japanese …..adults Pancreas”.JACC Study Group.,vol. 37 ,no.1,pp. 25–….30, 2008.
- Luo, M. Inoue, M. Iwasaki, S. Sasazuki, T. Otani, W. Ye ……and S. Tsugane, “Green tea and coffee intake and risk of …..pancreatic cancer in a largescale, population-based cohort …..study in Japan” (JPHC study). Eur. J. Cancer Prev., …..vol.16, no. 6, pp. 542–548, 2008
- Zhang, X. Zhao, X. Zhang, C. D’Arcy and J. Holman, …..”Possible protective effect of green tea intake on risk of ……adult leukemia”. Br. J. Cancer, vol. 98, pp.168–170, 2008.
- C.L. Sun, J.M. Yuan, W.P. Koh and M.C. Yu, “Green tea, ……black tea and breast cancer risk: a meta-analysis ……epidemiological studies”. Carcinogenesis, vol .27, no.7, ……pp.1310–1315, 2006.
- Zhang, C.D. Arcy, J. Holman, J.P. Huang and X. Xi, ….”Green tea and the prevention of breast cancer: a case–…..control study in Southeast China”. Carcinogenesis, vol. 28, …..no. 5, pp. 1074-1078, 2007.
- Clement, “Can green tea do that?” A literature review of the …….. clinical evidence. Prev. med., vol .49, pp. 83-87, 2009.
- [8] N. Kurahashi, S. Sasazuki , M. Iwasaki, M. Inoue and S. ….Tsugane, “Green tea consumption and prostate cancer risk in ….Japanese men” (JPHC Study Group). Am. J. Epidemiol., vol ….167, no.1, pp.71–77, 2008.
- Sasazuki, M. Inoue, T. Hanaoka, S. Yamamoto, T. ……Sobue and S. Tsugane, “Green tea consumption and ……subsequent of gastric cancer” (JPHC Study). Cancer ……Causes Control, vol. 15, no. 5,pp. 483–491,2004.
- Yang, X. O. Shu and H. Li, et al., “Prospective cohort ……study of green tea consumption and colorectal cancer risk ……in women”. Cancer Epidemiol. Biomarkers Prev., vol.16 …..,no.6,pp. 1219–1223,2007.
- [11] M. Shimizu, Y. Fukotomi and M. Ninomiya, et al., ” …..Green tea extracts for the prevention of metachromous …..colorectal adenomas”. Cancer Epidemiol. Biomarkers. Prev., …..vol. 17 ,no.11,pp. 3020–3025,2008.
- [12] R. Ide, Y. Fujino and Y. Hoshiyama, et al., “A prospective … ……….study of green tea consumption and oral cancer incidence in ……….Japan”.(JACC Study Group).Ann. Epidemiol., vol.17,no. ……….10,pp.821–826,2007.
- I.C. Arts, “A review of the epidemiological evidence on tea, ……flavonoids, and lung cancer”. J. Nutr., vol.138 ,no.3,pp.1561S–……1566S,2008.
- S.K. Myung, W.K. Bae and S.M. Oh, et al., “Green tea …..consumption and the risk of stomach cancer”. Int. J. Cancer,vol. 124 , no.3,pp. 670–677,2009.
- Y. Kakuta, N. Nakaya and S. Nagase, et al. , “Case-…..control study of green tea consumption and the risk of …..endometrial endometroid adenocarcinoma”. Cancer …..Causes Control,vol. 20 ,no.5,pp. 617–624,2009.
- V.W. Setiawan, Z.F. Zhang and G.P. Yu, “Protective effect …..of green tea on .the risks of chronic gastritis and stomach …..cancer”. Int. J. Cancer, vol.92, no. 4,pp. 600–604,2001.
- L.J. Su and L. Arab, “Tea consumption and the reduced …..risk of colon cancer–results from a national prospective …..cohort study”. Pub. Health Nutr., vol.5, no.3, pp.419–…..425, 2002.
- C.S. Yang, P. Maliakal and X. Meng, “Inhibition of …..carcinogenesis by tea”. Annu Rev Pharmacol Toxicol,vol. …..42,pp.25–54,2002.
- F.L. Chang, J. Schwartz, C.R. Herzog and Y.M. Yang, …..”Tea and cancer .prevention: studies in animals and …..humans”. J. Nutr., vol. 133, no.10, pp. S3268–.S3274, 2003.
- H. Matsumoto and T. Yamana, “green tea and cancer …..prevention” .J Women cancer,vol. 2 ,no.3,pp.152-158,2000.
- H.N. Graham, ” Green tea composition, consumption, and …..polyphenol chemistry”. Prev. Med., vol.21, no.3, pp. 334-…..350, 1992.
- Y. Kakuta, N. Nakaya and S. Nagase, et al., “Case-control …..study of green tea consumption and the risk of endometrial …..endometroid adenocarcinoma”. Cancer Causes …..Control,vol. 20 ,no.5,pp. 617–624,2009.
- V.W. Setiawan, Z.F. Zhang and G.P. Yu,” Protective …..effect of green tea on .the risks of chronic gastritis and …..stomach cancer”. Int. J. Cancer, vol. 92, no. 4,pp. 600–…..604,2001.
- V. Crespy and G. Williamson, “A review of the health …..effects of green tea catechins in in vivo animal models” J …..Nutr.,vol. 134, no.34, pp.31–40S, 2004.
- S.W. Huh, S.M. Bae and Y.W. Kim, et al., “Anticancer …..effects of epigallocatechin-3- gallate on ovarian carcinoma …..cell lines”. Gynecol. Oncol., vol. 94, no.3,pp. 760-768,2004.
- M.S. Han, J.S. Han, Y.K. Park, H. S. Lee, J.D. Park and …..C.K. Huh, “A Case of Metastatic Ovarian Cancer Arising in …..Plasma Cell Leukemia”. Korean J Obstet. Gynecol., vol.41 ….., no. 2, pp.602-606,1998.
- S.C. Larsson and A. Wolk ,” Tea consumption and ovarian …..cancer risk in a population-based cohort”. Arch Intern Med., …..vol.165, pp. 2683–6, 2005.
- M. Zhang, A.H. Lee, C.W. Binns and X. Xie, “Green …..tea consumption.enhances survival of epithelial ovarian …..cancer”. Int. J. Cancer, vol.112, no.3,pp. 465–469,2004.
- T. Sugiyama, Y. Sadzuka, A. Miyagishima, Y. Nozawa, K. …..Nagasawa, N. Ohnishi and T. Yokoyama, “Membrane …..transport on tumor cell and antitumor activity of …..doxorubicin and pirarubicin”. Drug Delivery System, vol. …..13,pp. 35- 40,1998.
- M. Zhang, C .W. Binns and A.H. Lee, “Tea consumption …..and ovarian cancer risk: a case control study in China”. …..Cancer Epidemiol. Biomarkers Prev., vol.11, no. 8,pp. 713–…..718,2002.
- T. Byers, J. Marshall, S. Graham, C. Mettlin and M. …..Swanson, “A.case-control study of dietary and nondietary …..factors in ovarian cancer “.J. Natl Cancer Inst.,vol.71, …..no.4, pp.681-686, 1983.
- D.R. Miller, L. Rosenberg and D.W. Kaufman, et al., …..”Epithelial ovarian cancer and coffee drinking”. Int. J. …..Epidemiol., vol. 16,no.1,pp. 13-17,1987.
- C. La Vecchia, E. Negre, S. Franceschi, B. D’Avanzo …..and P. Boyle, ” Tea.consumption and cancer risk”. Nutr. …..Cancer,vol. 17,no.1,pp.27-31,1992.
- H. Kuper, L. Titus-Ernstoff, B.L. Harlow and D.W. …..Cramer, “Population based study of coffee, alcohol and …..tobacco use and risk of ovarian.cancer”. Int. J. Cancer,vol. …..88,no.2,pp.313-318,2000.
- A. Tavani, S. Gallus and L. Dal Maso, et al., ” Coffee and …..alcohol intake .and risk of ovarian cancer: an Italian case-…..control study”. Nutr. Cancer,vol. 39,no.1,pp.29-34,2001.
- W. Zheng, T.J. Doyle, L. H. Kushi, T.A. ellers, C.P. Hong …..and A.R. Folsom, “Tea consumption and cancer incidence in …..a prospective cohort study of postmenopausal women”. Am. …..J. Epidemiol.,vol. 144,no.2,pp. 175-182, 1996.
- J.M. Nicolas, M.N. Clifford and C. Ionnides, …..”Consumption of tea modulates the urinary excretion of …..mutagens in rat treated with IQ. Role.of caffeine”. …..Mut.RES./ Gen.Toxcol.Enviro.Mut., vol.441 ,no.2 ,pp. …..191203,1999.
- S.S. Moshref, F. A. Khorshid and S.Y. Jamal, “The Effect …..of PM 701 on Mice Leukemic Cells:I-Tissue Culture Study …..of L1210 (In Vitro) II- In Vivo Study on Mice”. JKAU: …..Med. Sci., vol.13,no.1,pp. 3-19,2006.
- N. Carlos, L.A. Sherry, C.L. Norma and A.K. James , …..”Ovarian Rhadomyosarcoma presenting as Leukemia” ……Cancer, vol.52,pp. 297-300,1983.
- J.K. Lin , “Cancer chemoprevention by tea polyphenols …..through..modulating signal transduction pathways”. Arch. …..Pharm. Res.,vol. 25,no.5,pp. 561–571,2002.
- N. Ahmad, et al., “Green tea constituent’s …..epigallocatatechin-3,gallate.and induction of apoptosis and …..cell cycle arrest in human ,,,carcinoma cells”. J. Natl Cancer …..Inst.,vol. 89,pp.1881-1886,1997.
- J.F.R. Kerr, A. H. Wyllie and A.R.. Currie, ” Apoptosis: a …..basic biological .phenomenon with wide-ranging …..implication in tissue kinetics”. Brit J Cancer,vol. 68,pp. 239-…..257,1972.
- F. Spinella, V. Rosano Di Casto, S. Decandia, A. Albani, …..M.R. Nicotra,. P.G. Natali and A. Bagnato, ” Green tea …..polyphenol epigallocatechin-3- gallate inhibits the …..endothelin axis and downstream signaling pathways in …..ovarian carcinoma”. Mol. Cancer Ther. ,vol. 5,pp. 1483-…..1492,2006.
- Y.P. Lu, et al., “Inhibitory effect of black tea on the …..growth of established.skin tumor size, apoptosis, mitosis and …..bromodeoxyuridine incorporation into DNA”. …..Carcinogenesis,vol. 18,pp. 2163–2169,1997.
- P.R. Ber, “Apoptosis-the cell´s silent exit”. Life Sci., …..vol.59,pp. 369-78,1996.
- Y. Ren and J. Savill, “Apoptosis: the importance of being …..eaten”. Cell Death Diff., 5:563-568, 1998.
- L. Holmgren, A. Szeles, E. Rajnavolgyy, J. Folkman, G. …..Klein, I. Ernberg and K.I. Falk , ” Horizontal transfer of …..DNA by the uptake of apoptotic.bodies”. Blood,vol. 93,pp. …..3956-3963,1999.
- C.Aaij and P. Borst, “The gel electrophoresis of DNA”. …..Biochim. .Biophys Acta., vol.269,pp. 192-200,1972
- Y.Gavrieli, Y. Sherman and S.A.Ben Sasson, …..”Identification of programmed cell death in situ via specific …..labeling of nuclear DNA fragmentation”. J Cell Biol., …..vol.119, pp. 493-501, 1992.
- T. Sugiyama and Y.Sadzuka, “Combination of theanine …..with doxorubicin inhibits hepatic metastasis of M5076 …..ovarian sarcoma.Clin”. Cancer Res., vol. 5, no.2, pp. 413- …..416, 1999.
- M. Zhang, C.W. Binns and A. H. Lee, “Tea consumption …..and ovarian cancer risk: a case control study in China”. …..Cancer Epidemiol. Biomarkers Prev., vol. 11,no.8,pp.713–…..718,2002.
- B. Halder, U. Bhattacharya, S. Mukhopadhyay and A.K. …..Giri, “Molecular mechanism of black tea polyphenols …..induced apoptosis in human skin cancer cells: involvement …..of Bax translocation and mitochondria mediated ,,,,,death.cascade “Carcinogenesis, vol. 29, no.1, pp. 129 ,…138,2008.
- Y.P.Lu, Y.R. Lou, X.H. Li, J.G. Xie, D.Brash, ……M.T. Huang and A.H.Conney, “Stimulatory effect of oral …..administration of green tea or caffeine on ultraviolet light-…..induced increases in epidermal wildtype , p53, …..p21(WAF1/CIP1), and apoptotic sunburn cells in SKH-1 …..mice”. Cancer Res., vol.60, pp. 4785-4791,2000.
- K. Hastak, M.K. Agarwal, H. Mukhtar and M. …..L.Agarwal, “Ablation of either p21 or Bax prevents p53-…..dependent apoptosis induced by green tea polyphenol …..epigallocatechin-3- gallate”. FASEB J.,vol. 19,no.7,pp. 789-…..791,2005.
- Y.D. Jung and L.M. Ellis, “Inhibition of tumour invasion …..and angiogenesis .by epigallocatechin gallate (EGCG), a …..major.component of green tea”. .Int. J. Exp. Pathol., vol. …..82, .no.6,pp..309-316, 2001.
- C. Yihai and C. Renhai, “Can green tea minimize cancer …..risk? “Nature, vol.398, pp. 381-382,1999.
- J.S.Howell, J. Marchant and J.W.Orr, “The induction of …..ovarian tumors in mice with 9:10- Dimethyle-1:2- …..Benzanthercene”. British J.cancer, vol.4, pp.635-646, 1954.

This work is licensed under a Creative Commons Attribution 4.0 International License.





