How to Cite | Publication History | PlumX Article Matrix
Ajay Pratap Singh* and K. D. Sexena
Department of Chemistry, Bareilly College, Bareilly - 243 001 India
Correspondin Author E-mail: ajayojas@ yahoo.co.in
ABSTRACT: A Lectin was isolated from the seeds of Momardica charantia L.var. Jhalri (MCJ) using ammonium sulphate fractionation at pH 6.8 and 4°C. The purification of isolated lectin was done by ion exchange chromatography using DEAE-Cellulose with discontinuous gradient of acetate buffers. Purity and homogeneity of MCJ lectin was confirmed by 10% SDS-PAGE at pH 8.6. The same technique revealed its apparent molecular weight of 128kD indicating it to be a high mol. wt. lectin among the members of Cucurbitaceae family. MCJ lectin showed potent heamagglutination activity for O+ erythrocytes. The total lectin isolated was 118.80 mg per 100 g seeds. Climatic and ecological factors under which the plant grows up were assumed to be responsible for high yield of MCJ lectin.
KEYWORDS: Lectin; SDS-PAGE; Haemagglutination; Cucurbitaceae
Download this article as:| Copy the following to cite this article: Singh A. P, Sexena K. D. Lectin from the Seeds of Wild Bitter Gourd (Momardica Charantia L. Var. Jhalri): Isolation and Partial Charactrization. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Singh A. P, Sexena K. D. Lectin from the Seeds of Wild Bitter Gourd (Momardica Charantia L. Var. Jhalri): Isolation and Partial Charactrization. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9829 |
Introduction
Lectins are a class of proteins of non immune origin that possess at least one non-catalytic domain that specifically and reversibly bind to mono or oligosaccharide [1-2]. These proteins can agglutinate cells and precipitate glycoconjugates [3]. Lectins are very extended group of proteins present in plant, animals and microorganism. Plant lectins have been isolated from all parts of plant such as seeds, bark, bulbs, corns, fruits, leaves, root and flowers etc. but seeds are major source [4]. The specific interaction of lectins with glycoconjugates in solution or at cell surface makes these molecules valuable tool in biomedical sciences and biotechnology for structural analysis of glycoconjugates. In recent years some innovative lectins have come into existence with advanced studies [5-8]. The isolation of plant lectin seems to be mono polarized as large varieties of plant lectin have been studied from family Leguminoseae. Cucurbitaceae has become the area of interest among other families in the modern era as the fruits of this family are consumed in our daily diet. In the present study we have isolated and partially characterized a lectin from seeds of wild bitter gourd (Momardica charantia L.var. Jhalri) from the same family.
Material And Methods
Plant
The plant Momardica Charantia L. var. Jhalri (MCJ) is a member of Cucurbitaceae family and is a wild bitter gourd. The plant is generally found in Taraian region near dense forests of East – Northen India. The fruits of plant were collected from dense forest ‘Mahof’ in Pilibhit district of U.P. The matured plant fruits and their seeds were identified in the department of Bareilly College Braeilly.
Extraction of MCJ lectin
100 g matured seeds of MCJ were dried, dehulled, and afterwards powdered in home kitchen grinder. The seed flour was delipidated with petroleum ether (60- 80°C) in a soxhlet apparatus. Delipidated seed flour ( 50g) was extracted with 300 ml of 0.15M saline solution at pH 6.8 with 10% HCI , stirred at 4°C for 10 min in Sorvall refrigerated centrifuge RC plus. The crude seed extract was 60 % saturated with ammonium sulphate [9]. The precipitated protein was removed by centrifuging the fraction at 10000 rpm and 4°C for 20min, followed by exhaustive dialysis against triply distilled water. Dialysed protein was loaded on to a DEAE-Cellulose (AR, Whatman England) column of 2× 30 cm and equilibrated with 0.03M acetate buffer, pH 4.6. Elution of different fraction was carried out by using discontinuous gradient with 0.1M, 0.2M, and 0.3M acetate buffers each of pH 5.0. Each fraction was monitored for absorption at 280 nm in Beckmann D.U. spectrophotometer for detection of protein [10].
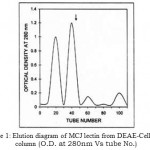 |
Figure 1: Elution diagram of MCJ lectin from DEAE-Cellulose column.
|
(O.D. at 280nm Vs tube No.)
Biological activity
To detect the biological activity of MCJ lectin each fraction after UV absorption was directly applied for haemagglutination assay. This was done first qualitatively and macroscopically on glass slides using ABO human erythrocytes and that of rabbit, sheep, goat, pig, buffalo and dog collected from IVRI, Izzatnagar Bareilly.
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
It was carried out on a vertical slab gel apparatus (Genei Pvt Ltd, Bangalore) according to Laemmli [11] using 10% acrylamide (AR, SRL Mumbai) at pH 8.6 under reducing and non-reducing conditions both. The electrophoresis was conducted at room temperature in constant voltage mode at 50 volts for 1hr. and afterwards at 100-110 volts. Protein bands were visualized by staining gel with Coomassie Brilliant blue R-250 (SRL Mumbai). Determination of mol. wt. of MCJ lectin was done by using broad range molecular weight markers (PMW-H Genei Pvt Ltd, Bangalore). (Fig 2A & 2B)
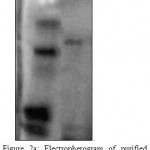 |
Figure 2A: Electropherogram of purified MCJ lectin on10% polyacrylamide gel at pH 8.6 under non reducing condition.
|
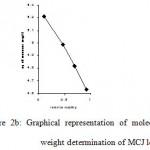 |
Figure 2B: Graphical representation of molecular FIG (2B) Graphical representation of molecular.
|
Lane
Broad range molecular weight marker (from top to bottom) containing:
Myosin (205kDa), Phosphorylase b (97.4 kDa), BSA (66kDa), Ovalbumin (43kDa).
Lane
Purified MCJ Lectin.
Protein Estimation
The estimation of total purified protein was done by Lowry’s method [12]. For this purpose Standard BSA (5mg/ml) was used from protein estimation kit Cat≠ KT-18, Genei Pvt Ltd, Bangalore.
Result And Discussion
During chemical extraction, the protein precipitated when crude seed extract was 60% saturated with solid ammonium sulphate. After dialysis the protein was purified by ion-exchanger chromatography. Elution by a discontinuous gradient resulted in the purification of MCJ lectin corresponding to peak II (Fig 1).The protein agglutinated O+ human erythrocytes strongly confirming its biological activity. The purity and homogeneity of MCJ lectin was justified by SDS-PAGE which showed a single band under non-reducing condition indicating the presence of MCJ lectin. Mol. wt. (Mr) of which was found to be 128 kDa. This one is a high mol. wt. as compared to other lectins from the same family e.g. Luffa acutangula (Mr 48 kDa), Cucubita maxima (Mr 48 kDa) , Cucurbita pepo (Mr 20 kDa) , Trichosanthes cucumerina (Mr 69 kDa), Trichosanthes dioica (Mr 55kDa), Trichosanthes anguina (Mr 45 kDa) and other Momardica Charantia species ( Mr 26 kDa) [13-18]. It is due to glycoprotein nature of MCJ lectin [19-21] and suggests that the protein may have high content of carbohydrate moieties. Under reducing condition with 2- merceptoehtanol, a new band corresponding to Mr 32 kDa was observed. It reveals tetrameric nature of MCJ lectin and may contain four identical subunits, linked by disulphide bridges. The need of heating protein sample before running gel to get tetrameric nature of MCJ lectin suggests that subunits may also be associated with non-covalent interactions such as hydrophobic interactions or/and hydrogen bonds. The total MCJ lectin isolated was 118.80 mg per 100 g of seeds of Momardica charantia L. var. Jhalri. This one is a high yield lectin among other species from same family except Trichosanthes cucumerina (350 mg lectin per 100 g seeds) [17]. The high level of concentration of MCJ lectin may be due to climatic and ecological conditions [22] because this particular species grow in Taraian regions of dense forests where extensive humid soil may be one of the factors in the development and accumulation of MCJ lectin in seeds. Since MCJ is a rich source of lectin, it may be used as an option for large production of lectin.
Acknowledgement
Authors are thankful to principal, Bareilly College Bareilly for providing research facilities, Dr Praveen Singh, Senior scientists, IVRI Bareilly, Prof. Y. D. Sharma Head Biotech, AIIMS Delhi and Dr. S. M. Tarique, Dean Academic affairs PGIE Bareilly for their valuable assistance and suggestions.
References
- Carlini,C.R., Grossi-de-Sa, M. F., Toxicon, 40, 1515-1539 (2002).
- Peumans, W. J., Van Damme, E. J. M., Plant Physiol., 109, 347-352 ( 1995).
- Sharon, N., Lis, H., in: Lectin, Chapman & Hall, New Yark, 2-65 (1989).
- Goldstein, J., Hays, C. E., Adv. Carbohydr. Chem. Biochem., 35, 127- 340 (1978).
- George, M. A., Bhide, S. V., Thengane, R. J., Hosseini, G. H. and Manjaya, J. G., Plant Breeding, 127, 150–153 (2008).
- Tsuruta, S.N.,Kishimoto, Y.,Nisimura, T. and Suda , Y., Biochem, 143 (6), 833-839 (2008).
- Kuku, A., Odekanyin, O., Adeniran, K., et al., African Journal of Biochemistry Research, 3 (6), 272-278 (2009).
- Rahaie, M. and. Kazemi, S.S., Biotechnology, 9, 428-443 (2010).
- Green, A. A., Hung, W. L., Methods In Enzymology, 1, 67-90 (1955).
- Singh, A. P., Effect of chemical and environmental factors on chemical composition and biological activity of lectins from family Cucurbitaceae. Ph.D. Thesis., J. P. Rohilkhand University UP, India (2007).
- Laemmli, U. K. Nature., 227, .680 (1970).
- Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J., J. Biol. Chem.,193, 265-275 (1951).
- Anantharam, V., Patanjali, S. R., Swamy, M. J., Sanadi, A. R., Goldstein, I. J., Surolia, A., J. Biol. Chem., 261 (3), 14621-14627 (1986).
- Read, J. M., Northcote, D. H., Eur. J. Biochem., 134 (3), 561-569 (1983).
- Allen, A. K., Biochem. J., 183, 133-137 (1979).
- Padma, P., Komath, S. S., Nadimpalli, S. K., Swamy, M. J., Phytochemistry, 50, 363-371 (1999).
- Anuradha, P., Bhide, S. V, Phytochemistry, 52, 751-758 (1999).
- S. –L.Li, Experientia, 36, 524-527 (1980).
- Glossman, H. and Neville, D. M., J. Boil. Chem., 246, 6339-6346 (1971).
- Schubert, D., J. Mol. Biol., 51, 287-301 (1970).
- Leach, B. S., Collawn, J. F. and Fish, W. W., Biochemistry., 19, 5741-5747 (1980).
- Rego, E. J. L., Carvalho, D. de, Marangoni, S., et al., Phytochemistry., 60, 441-446 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.





