How to Cite | Publication History | PlumX Article Matrix
Niraj Ghanwate1*, P. V. Thakare1, P. R. Bhise2 and A. Wandhre1
¹Department of Biotechnology, Sant Gadge Baba Amravati University, Amravati - 444 602 India.
²Department of Microbiology, Dr. P D M Medical College, Amravati - 444 603 India.
Corresponding Author E-mail: nirajghanwate@gmail.com
ABSTRACT: Objective-The Extended Spectrum Beta Lactamases (ESBL) producing bacteria are increasingly causing urinary tract infection (UTI) both in hospitalized and outpatients. Failure to identify them also contributes to their uncontrolled spread. Therefore, identification of the resistant phenotypes is important, particularly in developing countries where excessive use of antibiotics and lack of adequate antimicrobial resistance surveillance is there. The present study was conducted with an objective to examine the incidence of ESBL producing stain of Klebsiella pneumoniae and E. coli from UTI. Materials and methods-A total of 200 urine samples were analyzed during the period of study. Isolates of E. coli and Klebsiella pneumoniae were selected for further study. These isolates were tested for susceptibility to antibiotics like cephotaxime, chloramphenicol, ciprofloxacin, gentamycin and amoxicillin. ESBL production was tested by double disk synergy test recommended by Clinical and Laboratory Standards Institute. Result- Out of 200 urine samples, 77 and 58 isolates were E.coli and Klebsiella pneumoniae respectively. Among these uropathogens, 45.45% of E. coli and 31 % of Klebsiella pneumoniae were ESBL positive. ESBL producing strains were also found to be resistant to other antibiotics tested. Conclusion- The investigation has demonstrated that it is important for clinical microbiology laboratories to have the ability to detect a report on ESBL production in uropathogens of Gram-negative bacteria particularly with Klebsiella pneumoniae and E.coli isolated from urinary tract infection.
KEYWORDS: UTI, E.coli; Klebsiella pneumoniae; ESBL
Download this article as:| Copy the following to cite this article: Ghanwate N, Thakare P. V, Bhise P. R, Wandhre A. Occurrence of Extended Spectrum B-Lactamases Among E. Coli and Klebsiella Pneumoniae Isolated from Urinary Tract Infection from Tertiary Health Care Center in Amravati District. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Ghanwate N, Thakare P. V, Bhise P. R, Wandhre A. Occurrence of Extended Spectrum B-Lactamases Among E. Coli and Klebsiella Pneumoniae Isolated from Urinary Tract Infection from Tertiary Health Care Center in Amravati District. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9836 |
Introduction
Extended Spectrum of b Lactamases (ESBL) are the derivatives of common b lactamases that have undergone one or more amino acid substitutions near the active site of the enzyme, thus increasing their affinity and the hydrolytic activity against third generation cephalosporin and monobactams. Extensive use of newer generation cephalosporin has been the strong factor for the evolution of ESBLs. The ESBL are encoded by transferable conjugative plasmids, which often code resistance determinants to other antimicrobial agents such as aminoglycosides. (1, 2) ESBL producing strains of Enterobacteriaceae have emerged as a major problem in hospitalized as well as community based patients. (3, 4) Infections due to ESBL producers range from uncomplicated UTI to life threatening sepsis. Microorganisms responsible for urinary tract infections such as E. coli and Klebsiella pneumoniae have the ability to produce ESBLs in large quantities. (3) The prevalence of these uropathogens constitutes a serious threat to current b- lactam therapy leading to treatment failure and consequent escalation of cost. Occurrence of ESBL producing Klebsiella spp. has been reported from South India (5) and Central India. (6) Recent reports have high lightened the emergence of ESBL producing strains with a wide spectrum of antibiotic resistance including trimethoprim, amikacin, streptomycin and gentamycin.(7) The present study was conducted with an objective to examine the incidence of ESBL producing strains of E. coli and Klebsiella pneumoniae recovered from UTI. Moreover, there are no reports detailing the evidence of ESBL producing uropathogenic E. coli and Klebsiella pneumoniae from this region.
Materials and Methods
Clinical isolates
A total of 200 urine samples were processed for significant bacteriuria from patients clinically suspected to have UTI. All E. coli and Klebsiella pneumoniae isolated from significant urine samples were included in the study.
Antimicrobial susceptibility testing
The sensitivity of the isolates to third generation cephalosporin viz; cephotaxime (30 mg) and to other antibiotics such as ciprofloxacin (5 mg), amoxicillin (10 mg), gentamycin (10 mg) and chloramphenicol (30 mg), (Hi-media, India) was determined by disc diffusion method. Susceptibility and resistance was determined based on interpretative criteria recommended by CLSI guidelines. (8) Isolates with resistance or with decreased susceptibility to third generation antibiotics were selected for the study.
Double disc synergy test for determination of ESBL activity (DDST)
ESBL production of the isolates by DDST was determined by using Hi- media ESBL identification test kit, (SD238). A disc of cephotaxime with clavulanic acid, (30/10 mg) was placed in between 30 mg disc each of cephotaxime and any other third generation cephalosporin at a distance of 30 mm apart on a lawn culture of resistant isolate under test on Mueller Hinton agar, (Hi- media). (9) The test organism was considered to produce ESBL, if the zone size around the test antibiotic disc increased towards the clavulanic acid disc. This increase occurs because clavulanic acid present in the disc inactivates the ESBL produced by the test organism.
Results
A total of 200 urine samples were analyzed during the period of study. Among 200 urine sample 150 samples had significant bacteriuria with the count more than 105 cfu/ml, 38 samples had no significant count and remaining 12 samples had no growth.
Out of 150 urine samples with significant count, E.coli was isolated in 51%, Klebsiella pneumoniae in 38%, Enterococcus faecalis in 6%, followed by Staphylococcus in 4% and Micrococcus in 1%. A total of 77 clinical isolates of E.coli and 58 clinical isolates of Klebsiella pneumoniae were isolated from 150 significant urine samples. The sensitivity profile of E. coli exhibited 57% resistance to cephotaxime, 65% to ciprofloxacin, 68% to amoxicillin and gentamycin and 60% to chloramphenicol. Klebsiella pneumoniae showed 62% resistance to cephotaxime, 66% to ciprofloxacin, 100% to amoxicillin, 59% to gentamycin and 69%to chloramphenicol. It was observed that all isolates of Klebsiella pneumoniae were resistant to amoxicillin. Antimicrobial susceptibility results of isolated E. coli and K. pneumoniae are shown in figures- 1 and 2 respectively.
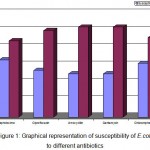 |
Figure 1: Graphical representation of susceptibility of E.coli to different antibiotics
|
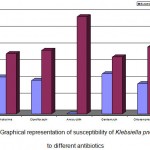 |
Figure 2: Graphical representation of susceptibility of Klebsiella pneumoniae to different antibiotics.
|
ESBL production by Double Disc Synergy Test was carried out on 135 isolates of E. coli and K. pneumoniae. Among them 53 (43%) isolates were identified as potential ESBL producers. The zone of inhibition of cephotaxime alone was compared with the zone of inhibition in combination with clavulanic acid. According to CLSI recommendations a difference of >5mm increase in zone diameter for cephotaxime with clavulanic acid versus its zone diameter when tested alone confirms the presence of ESBL. The isolates of E. coli and Klebsiella pneumoniae that were considered positive for ESBL production exhibited a clear difference of >5mm in zone diameter to cephotaxime and cephotaxime with clavulanic acid. Figure 3 demonstrates the result for ESBL production. From the 77 isolates of E. coli 35 (45.45%) were ESBL positive and from the 58 isolates of Klebsiella pneumoniae 18 (31.0%) isolates were ESBL positive (Table-1).
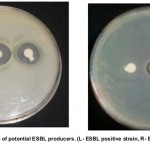 |
Figure 3
|
Table 1: Identification of ESBL positive isolates.
| Organisms | No. of isolates | % of ESBL isolates |
| E. coli
Klebsiella pneumoniae |
77
58 |
35 (45.45%)
18 (31.0%) |
Discussion
ESBL producing organisms have become an important clinical problem due to their resistance to multiple antibiotics. Thus antibiotic options in the treatment of these organisms are extremely limited. β- Lactam antibiotics are usually used for treatment. Detection of ESBL production is important because it is recommended that any organism confirmed for ESBL production according to CLSI criteria should be reported as resistant to all extended spectrum b lactam antibiotics.
Clinical laboratories lack the awareness of importance of ESBL. They need to develop quick screening methods to assess the mechanism of b lactam resistance in their isolates. As detected in our study, resistance to cephotaxime would suggest an ESBL and combination with clavulanic acid bringing it back to complete susceptibility confirms an ESBL. The occurrence of ESBL producers in urinary isolates of E. coli and Klebsiella pneumoniae in our study was found to be 45% and 31% respectively. Babypadmini et al (2004) have shown 40% and 41% ESBL positivity among K. Pneumonia and E.coli respectively in their study. Previous studies from India have reported prevalence of ESBL producers to be 66 to 68%. ESBL production was reported among Gram negative bacteria from tertiary care hospital by Mathur et al (2002). Tankhiwale et al (2004) reported that 48% of urinary isolates tested were ESBL producers. In present study it was 43%.
ESBL production also coexisted with resistance to several other antibiotics. It was demonstrated that there was high incidence of resistance to other antibiotics among ESBL producing E. coli and K. pneumoniae UTI isolates. Present study revealed that ESBL were predominantly present among E. coli (45%) as compared to Klebsiella pneumoniae (31%). Similar findings were also demonstrated by Kumar et al (2006). The study conducted by Taneja et al (2008) the highest positivity was observed in Klebsiella (51.2%), followed by the E.coli (40.2%).
Therefore it is necessary and useful to perform screening and confirmatory test for phenotypic detection of these organism in a routine laboratory diagnostic work. All the ESBL producers are resistant to may classes of antibiotics resulting in limited treatment option. Treatment of infection due to these organisms could be difficult and complex.
References
- Bal S., Hospital today. , 5, 96- 101 (2000).
- Subha A. and Ananthan S., Indian J Med Microbiol. , 20, 92-95 (2002).
- Chaudhary U. and Aggarwal R., Indian J Med Microbiol. , 22, 75-80 (2004).
- Rodriguez- Bano J., Navarro M. D., Romero L., Martinez- Martinez L., Munian M.A., Perea E. J., et al. , J. Clin. Microbiol. , 42, 1089- 94 (2004).
- Abigail S. Mathai E., Jesudasan M. V., John T. J., Indian J Med Res., 102, 53-5 (1995).
- Hansotia J. B., Agarwal V., Pathak A. A., Saoji A. M., Indian J Med Res., 105, 158-61(1997).
- Laura V., Pezzella C., Tosini F., Visca P., Petrucca A., Carattoli A., Antimicrob Agents Chemother., 44, 2911-14 (2000).
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA, (2006).
- Kumar M. S., V Lakshmi, R Rajgopalan.,Indian J Med Microbiol, 24, 208-11(2006).
- Babypadmini S., B Applaraju. , Indian J Med Microbiol, 22, 172-174 (2004).
- Mathur P., Kapil A., Das B., Dhavan B., Indian J Med Microbiol. , 115, 153-7 (2002).
- Taneja Neelam, Meera Sharma. , Indian J Med Research, (2008).
- Tankhiwale S.S., Jalgaonkar S.V., Ahamad S., Hassani U. , Indian J Med Res, 120, 553-6 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.





