How to Cite | Publication History | PlumX Article Matrix
Secondary Metabolite Profiling of Acacia concinna
Sandip Todkar, Rohit Todkar, Vishal Kavathekar, Shantanu Kulkarni and Arvind Kulkarni
Department of Biotechnology, Department of Microbiology, Gogate - Jogalekar College,University of Mumbai, Ratnagiri - 415 612 India.
Corresponding Author E-mail: sandip_todkar7@rediffmail.com
ABSTRACT: Secondary metabolites are a wide range of compounds from different metabolite families. Secondary metabolites are often involved in plant protection and which do not participate directly in growth and development. The aim of our study was to profile the potent secondary metabolites responsible for the antibacterial and antifungal activity. Secondary metabolites consist of varieties of chemical compounds with different structures and chemical properties. In order to get overview of secondary metabolites and to detect potent compounds responsible for the antibacterial and antifungal activity from Acacia concinna, a well suited profiling technique: Extraction with different chromatographic techniques (GC-MS, HPLC-PAD/MS) was done. The polar and non-polar methanol extracts of Acacia concinna were collected by using a suitable technique and the respective residues were subjected for HPLC-PAD/MS and GC-MS analysis. These chromatographic methods analyses ionic (charged) compounds such as Alkaloids, and non-ionic (neutral) compounds such as Flavonoids, Saponins, Tannins, Gums and Mucilage and Phenolic compounds. The profiling method reveals presence of a series of sequential compounds like 1-Methylimidazol-5-carboxaldehyde, Propanoic acid, 2- Octanamine, 3-Haptenoic acid, 3(2)-Furanone, 2-Butenedioic acid, 4-Hapten, Butanoic acid 2-propenyl ester, Pentadecanoic acid. These compounds show negative impacts on many microorganisms with its all respects. Thus it was concluded that Acacia concinna can be used as chemotherapeutic or Medicinal purpose.
KEYWORDS: Alkaloids, Flavonoids; GC-MS; Acacia concinna; Secondary Metabolites; HPLC-PAD/MS
Download this article as:| Copy the following to cite this article: Todkar S, Todkar R, Kavathekar V, Kulkarni S, Kulkarni A. Secondary Metabolite Profiling of Acacia concinna. Biosci Biotech Res Asia 2011;8(1). |
| Copy the following to cite this URL: Todkar S, Todkar R, Kavathekar V, Kulkarni S, Kulkarni A. Secondary Metabolite Profiling of Acacia concinna. Biosci Biotech Res Asia 2011;8(1). Available from: https://www.biotech-asia.org/?p=21235 |
Introduction
The most diverse group of Secondary metabolite compounds occurring in many plant species and are responsible for the responses to stresses. Secondary metabolites are not required for the cell growth and maintenance but used against most of the biotic and abiotic forms. (Ujjwala J. Supe, 2007). Some secondary metabolites are also involved in cell pigmentation in flower and seed which attracts seed pollinators and seed dispensers, so also involved in plant reproduction (Winkel-Shirley B. et al. 2001). In the recent study plant secondary metabolites carry much more attention with respect to presence of certain chemical compounds that would be helpful in medical application. (Ravindra Kumar, et al., 2011). Potential antibacterial activity of various extracts of Acacia concinna in different organic solvents was studied. (P. Sampatkumar, et al., 2008). In the previous findings it was found that Acacia concinna has antibacterial and antifungal activity and also the presence of secondary metabolites. (Todkar S S, et al., 2010).
Phytochemical screening was done so as to detect the presence of secondary metabolites. (E. A. Hussein, et al., 2010) Even plant secondary metabolites also have certain chemical and pharmaceutical properties that are useful for human health (Raskin I, et al., 2002; Reddy L, et al., 2003). Compounds like Flavonoids, Terpenoids and Alkaloids are recently used as drugs or dietary supplements to prevent various diseases (Reddy L, et al., 2003), and even though it is used to inhibit cancer (Watson A, et al., 2001; Reddy L, et al., (2003). Secondary metabolites have some specific chemical properties which needs specific extraction and analysis methods, (polar to non-polar). It is possible to account probable number of compounds by using different analytical methods coupled with various extraction methods. (Rispal N, et al., 2005).These compounds were extracted in various solvents like methanol, benzene, chloroform, acetone, petroleum ether and aqueous extracts from water soluble to non soluble compounds, and non polar compounds by using dichloromethane. Then it is possible to separate by chromatographic methods (ion exchange and reverse phase) and to analyze fractions by GC-MS and HPLC-PAD/MS. The main objective of this study was to profile each and every secondary metabolite responsible for the antibacterial and antifungal activity from Acacia concinna. (Rispal N, et al., 2005).
The profiling method was described here was originally designed by Molecular Nature Ltd.( International Patent application numbers, (PTC/GB 2003/000905, PCT/GB 200300880, PCT/GB 200300892, PCT/GB 200300906) in order to identify and isolate new compounds which is having commercial values (Watson et al., 2001; Reddy et al., 2003). Thus it allows detection of large numbers of compounds from this plant species.
Materials and Methods
Collection of Plant Sample
The pods of Acacia concinna were collected from agricultural fields of Maharashtra, India (October 2010). The plant was identified and confirmed using standard manuals (Theodore Cooke, Flora) and used for further analysis.
Secondary Metabolite profiling procedure
The process described below is set up for the Perkin Elmers’s GC-MS system (model Q-Mass 910; Perkin Elmer, UK) and Water’s integrity HPLC-PDA-MS system (Water’s UK) with Millennium 32® software for data analysis. The procedures and consumables are described in protocols.
Secondary metabolites Extraction
Secondary metabolite consists of series of sequential extraction in various solvents like petroleum ether, benzene, acetone, methanol, chloroform, and in aqueous solvents to collect polar compounds and dichlomethane to collect non polar compounds, but in this study we have analyzed only methanol extract of pods Acacia concinna because methanol extract of pods Acacia concinna has maximum antibacterial and antifungal activity against Klebsiella pneumoniae, Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa, Fungus: Aspergillus niger, Penicillium spp. and Candida albicans. (Todkar S S, et al., 2010).
Polar Extraction
The pods of Acacia concinna was allowed to dry and pulverized by using mortar and pestle. 5 gm pulverized material was dissolved in 50ml of methanol solvent and kept in an orbital shaker overnight. The obtained extracts were filtered with Whattman No. 42 filter paper (125mm) and the filtrate was collected and used for experimental analysis. The residual material is then stored at 20 C and freeze dried before dichloromethane extraction. The extracted materials are then fractionized by ion exchange chromatography. Initially sample is applied to a 20×1 cm glass column containing 3 cm of Dowex 50X-X8 (HCL form Sigma) resin previously regenerated by adding excess 2N HCL soaking for 10 minutes, then washing it with deionised water until it reaches to neutral pH and finally equilibrated with 50 ml of all solvents. The unbound fraction of the sample, containing non ionic compounds, is collected in 500ml flask. The column was washed with ethanol and deionised water, the effluents are colleted, added to unbound fraction which is stored at 20 C before subsequent fractionation and analysis. After the washing the column is eluted with 200ml of ammonium hydroxide (NH4OH) and the effluent containing ionic compounds was collect in new flask. This fraction is then carefully evaporates at 370C using rotary evaporator up to 10 ml left in evaporator after that extracted material is transferred into 10ml glass vial and it is stored at 20 C before GC-MS analysis (Rispal,N, et al., 2005).
Non ionic Fractionation
The unbound fraction from the ion exchange chromatography previously collected is further scavenged by reverse phase chromatography using an HP 20 column on a Bioflash Chromatography system. Before the separation the HP 20 column is washed with 200ml of acetone and equilibrated with 20% methanol, then the unbound fraction is applied on the column and the HP 20 column is washed with 200ml acetone. The washed solution along with the unbound fraction containing mainly sugars and most of the hydrophilic Flavonoids were discarded (As they would mask all the others secondary metabolites present). The column is then eluted with 250ml of acetone in methanol (2:4, v/v). The effluent collected in one flask is then carefully evaporated at 370C using rotary evaporator evaporator up to 10 ml left in evaporator after that extracted material is transferred into 20ml glass vial, and it is stored at 20 C before HPLC-PDA/MS analysis (Rispal,N, et al., 2005).
Non Polar Extraction
The residual material from the polar extraction is then is placed in filter paper thimble inside a glass soxhlet apparatus, on other hand in 500ml flask containing 50ml of dichlomethane and 5-8 glass beads is placed on a heating mantle. The soxhlet is attached at top up of the flask and 50ml of dichloromethane is slowly added to sample. Then a refrigeration column is fixed to a soxhlet apparatus and the cooling water is turned on. The heating is switched on ensuring a study refluxing rate and left 8 hours form extract. At the end of the extraction the heating mantle is switched off and the soxhlet apparatus is allowed to cool before being dismantled. The dicholomethane extract is then added to 50ml of HP 40 resin in a one flask and the resin is completely dried with a rotary evaporator at 370C. Then it is transferred in 500 ml conical flask where it is eluted 5 times with 50ml, 5% acetone in methanol (1:10 v/v).The solution is then filtered through tissue paper in to 500 ml flask and evaporated up to 10 ml at 370C. The solution left is then transferred in 20 ml glass vial and flask is rinsed with few drops of 10% acetone in methanol (1:10, v/v) which are added to 10 ml vial. Then the vial is stored at 20 C and freeze dried before HPLC-PAD/MS analysis (Rispal, N, et al., 2005).
Table 1: Qualitative analysis of Secondary metabolites showing antimicrobial effect against various microorganisms.
| Sr. | Secondary | Klebsiella | Bacillus | Escherichia | Staphylococcus | Psedomonas |
| No. | Metabolites | pneumoniae | subtilis | coli | aureus | aeruginosa |
| 1 | 1Methylimidazol-5carboxaldehyde, | _ | _ | _ | _ | _ |
| 2 | Propanoic acid, | _ | _ | _ | _ | _ |
| 3 | 2- Octanamine | _ | _ | _ | _ | _ |
| 4 | 3-Haptenoic acid, | _ | + | + | + | + |
| 5 | 3(2)- Furanone, | _ | _ | _ | _ | _ |
| 6 | 2-Butenedioic acid, | _ | _ | _ | _ | _ |
| 7 | 4-Hapten, | _ | _ | _ | _ | + |
| 8 | Butanoic acid 2-propenyl ester | _ | _ | _ | _ | – |
| 9 | Pentadecanoic acid. | _ | _ | _ | _ | _ |
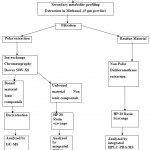 |
Figure 1: Flow chart of secondary metabolite profiling method.
|
Result
In this study the profiling of secondary metabolites from Acacia concinna was done. Thus, the method must be carefully chosen and utilized for profiling; previous work was done to screen out secondary metabolites from Acacia concinna (Todkar S S, et al. 2010) and now used to obtain an overview of the secondary metabolite contents of Acacia concinna in methanol extracts. The analysis report reveals that the majority of the antibacterial and antifungal activity of methanol extracts of pods of Acacia concinna is because of the presence of potent secondary metabolites. Thus, it supports previous findings with respect to its presence of antibacterial and antifungal activity. (Todkar S S, et al. 2010) Moreover, profiling furnishes the detection of novel secondary metabolites in terms of Flavonoids and Alkaloids observed in Pods of Acacia concinna. (Natarajan and Natrajan, 2009). These metabolites shows a series of sequential compounds like 1-Methylimidazol-5-carboxaldehyde, Propanoic acid, 2- Octanamine, 3-Haptenoic acid, 3(2)- Furanone, 2-Butenedioic acid, 4-Hapten, Butanoic acid 2-propenyl ester, Pentadecanoic acid which falls under categories of Alkaloids, Flavonoids, Phytosterols, Saponins, Tannins, Gums an and Phenolic compound.(Fig.2) Many of them have negative impacts on growth of microorganism. Alkaloids, Flavonoids can block ion channels, inhibit enzymes needed for metabolic activity and also interfere with neurotransmission producing hallucinations (Doyle DA, et al., 1998; Reddy L, et al., 2003). Certain Phenol derivatives can cause oxidative damage leading to death of microorganism. Propanoic acid inhibits the growth of mold and some bacteria by with making pores in the cell wall of many bacterias. Butyric acid can act as an HDAC inhibitor, inhibiting the function of histone deacetylase enzymes (Drummond DC, et al., 2005). The GC-MS analysis reveals that the presence of these compounds that can lead to form potential source of secondary metabolites, with better documented content. Each of these chemical compounds has a specific role with respect to inhibition of growth of microorganisms, and they simply interfere or arrest the metabolic activities of most of the microorganisms. Thus, it gives clear cut idea about novel secondary metabolites and their medicinal uses. (Rajasekaran, 2001). The Secondary metabolite profiling and antimicrobial activity is in support that higher plants represent a potential source of new anti-infective agents (De Smet, 1997; Cowan, 2001; Kelmanson et al, 2001; Srivasan et al, 2001). The GC-MS analysis and HPLC-PDA/MS analysis were carried out for the detection of secondary metabolites.
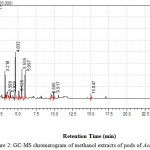 |
Figure 2: GC-MS chromatogram of methanol extracts of pods of Acacia concinna.
|
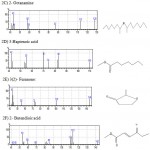 |
Figure 1a – h:
|
Conclusion
The study reveals the usefulness of medicinal plants in order to control the disease caused by most of bacterial species. Plants extracts assumed to have tremendous importance in medicine and health care industry. From the above study it was clear that the Acacia concinna (pods) has potent source of secondary metabolites having antibacterial activity in the chemical compounds present in it. The study entails the profiling of secondary metabolites with the presence of compounds, and these chemical compounds shown to have antibacterial activity with respect to inhibition and blocking of important enzymes required for the growth and metabolism of microorganisms. Purification of active compounds of pods of Acacia conicnna can be used as chemotherapeutic agent. Thus, from this study it was concluded that the antibacterial and antifungal activity of Acacia concinna is because of the presence of potent secondary metabolites which were profiled in this research so as to detect specific chemical compounds.
Acknowledgement
We are very much thankful to Department of Microbiology, Shivaji University Kolhapur, for their guidance and help.
Reference
- Ujjwala J. Supe (2007) “In vitro regeneration of Aloe barbadensis” 6(4): 601-603, 2007.
- Winkel-Shirley B. et al. (2001) “Flavanoid biosynthesis. A Colorful model for genetics, biochemistry, cell biology, and biotechnology”. Plant physiology 126,485-493.
- Ravindra Kumar, Gaurav Varma, Om Prakash et al., (2011). “Head space GC/MS analysis of Volatile Constituents of Trichilea cannoriades Wight and Arn. Extracts and there in vitro Antiplasmodium activity against Plasmodium falciparum isolates”. Research Journal of Phytochemistry. 5 (1): 41- 47, 2011.
- Sampatkumar, B. Dheeba, V. Vidyasagar et al., (2008) “Potential antibacterial activity of various Extracts of Bacopa monnieri” (Linn). International Journal of Pharmacology. 4(3): 230-232, 2008.
- Todkar S S, Chavan V V, et al, (2010) “Antibacterial Activity and Screening of Secondary Metabolites from Acacia concinna”. Journal of Pure and Applied Microbiology, Vol.4 (2), pg 815-818.
- A. Hussein, A. M. Taj- Eldeen et al., (2010). “Phytochemical screening, Total Phenolics and Antioxidant and Antibacterial Activities of Callus from Brassica nigra L. Hypocotyl Explants”. International Journal of Pharmacology. 6(4): 464-471.2010.
- Raskin I, Ribnicky DM, et al. (2002) “Plants and Human Health in the twenty-first century”. Trends in Biotechnology 20, 522-531.
- Reddy L, Odhav B, et al. (2003) “Natural Products for Cancer prevention: a global perspective”. Pharmacology and Therapeutics 99, 1-13.
- Watson AA, Fleet GWJ, et al, (2001) “Polyhydroxylated alkaloids-natural occurrence and therapeutic applications” .Phytochemistry 56, 265-295.
- Rispal, N., Robert Nash, et al. (2005) “Secondary Metabolite Profiling”. Lotus japonicus Handbook. 341-348.
- Doyle DA, Morais Cabral J, et al. (1998). “The structure of the potassium channel: molecular basis of K+ conduction and selectivity”. Science 280 (5360): 69–77.
- Drummond DC, Noble CO, et al. (2005). “Clinical development of histone deacetylase inhibitors as anticancer agents”. Annu. Rev. Pharmacol. Toxicol. 45: 495–528
- Rajsekaran P E (2001), “Medicinal Plant Sector in India”. 6, 23-31.
- De Smet P A G (1999), “The Role of Plant Derived Drugs and Herbal Medicines in Healthcare”, Drugs, Vol. 54, pp. 801-840.
- Cowan M M (1999), “Plant Products as Antibacterial agents”, Clin. Micobial. Rev.,Vol, 12, pp. 564-582.
- Srivasan D, Nathan, Suresh T and Perumalsamy O (2001), “Antibacterial activity f Certain Indian Medicinal Plant used in Folkloric MEDICINE,” J Ethannophamacol., 50, 27-34.

This work is licensed under a Creative Commons Attribution 4.0 International License.





