How to Cite | Publication History | PlumX Article Matrix
Study on Keratinophilic and Keratinolytic Fungi Isolated from Birds’ Feathers and Animal Hairs
K. Moorthy, I. Prasanna, S. Vimalan, V. Lavanya, A.T. Selvi, T. Mekala and N. Thajuddin
¹Department of Microbiology, Vivekananda College of Arts and Sciences for Women, Elayampalyam, Tiruchengode - 637 205 India.
²Department of Microbiology, Bharathidasan University, Tiruchirappalli, TamilNadu India.
Corresponding Author E-mail: moormicro@gmail.com
ABSTRACT: A total of 75 bird feathers and 22 animal hair samples (97 samples) were processed for invitro degradation of keratin. Both keratinophilic and keratinolytic fungi were identified and among them, 22 fungal genera were isolated which represented about 27 different species. All the 27 isolates were keratinophilic and among them 12 were found to have keratinolytic activity. Hen feather samples showed a high incidence of about 55 fungal isolates, followed by kaadai (Wild chicken) samples with 27 isolates, rabbit hair samples with 19, turkey and pigeon each with 18 isolates, 12 isolates were obtained from the goat hair samples, cow hair samples with 5 isolates and cat and dog hair with 2 isolates respectively. Among the 158 isolated fungal species, the genus Aspergillus was the found to be predominant. Among them, Aspergillus niger was predominant with 38 isolates followed by Fusarium spp. with 19 isolates and Scopulariopsis spp. was found to be in 11 isolates.
KEYWORDS: Keratinophilic; keratinolytic; hair bait; birds feathers; animal hairs
Download this article as:| Copy the following to cite this article: Moorthy K, Prasanna I, Vimalan S, Lavanya V, Selvi A. T, Mekala T, Thajuddin N. Study on Keratinophilic and Keratinolytic Fungi Isolated from Birds’ Feathers and Animal Hairs. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Moorthy K, Prasanna I, Vimalan S, Lavanya V, Selvi A. T, Mekala T, Thajuddin N. Study on Keratinophilic and Keratinolytic Fungi Isolated from Birds’ Feathers and Animal Hairs. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9485/ |
Introduction
Keratinophilic fungi are an ecologically important group of fungi that cycle one of the most abundant and highly stable animal proteins on earth. Keratin (from the Greek word means horn) is tough, fibrous insoluble material provides an outer coat that serves to prevent the loss of body fluids from animals. Keratin is one of the proteins of the family scleroproteins, which are the main constituents of the hair, nails and horny tissues. It has disulphide bonds with numerous cross linking. Due to the strength and stability of keratin very few organisms are able to break and utilize it. The biggest group of organism that can utilize keratin as sole carbon and nitrogen source are the Keratinophilic fungi (Rahul Sharma et al., 2003).
Hair is not solely made up of keratin and many keratinophilic fungi grow on natural hair using the non-keratin lipid fraction of the hair. Therefore, those fungi that are keratinophilic in nature should also be keratinolytic and degrade keratin. Keratinolytic fungi in the soil produced keratinase enzyme and lead to the degradation of the keratinized materials such as hides, furs, claws, nails and horns. Depending upon the origin, the keratin is always found associated with lipids of varied nature. These lipids, which can make keratin resistant to microbial degradation, are utilized as nutrients by keratinophilic fungi (Khanam, 2004). Production of lipolytic enzymes by keratinophilic fungi is important since they can assist them in initial colonization of natural keratin which proteases are acting upon the products of keratin hydrolysis leading to complete degradation of hydrolysate into aminoacids (Das and Banerjee, 1977; Kunert, 2000; Baxter and Trotter, 1969; Pugh and Evans, 1970 and Deshmukh et al.,1981)
Thus, keratin degradation is a multienzymic process. Earlier studies on the Keratin hydrolyzing enzymes of the keratinophilic fungi were mainly aimed to understand their role in development of mycotic infection in man and animals (Baxter, 1973 and Snider et al., 1993). Since the Keratinophilic fungi are soil borne saprophytes which might habitat on the wild vertebrates and birds that mainly expose to these types of fungi, which are able to produce infection in such animals and birds. Hence, the study of these keratinophilic fungi paves way to protect (or) save the animals and birds from the infection and also to take precautious measure for the contagious diseases (or) infections from animals to human beings.
The large amount of feathers produced and their localized accumulation create a serious disposal problem leading to environmental pollution. In several countries, industry uses incineration to dispose of poultry feathers and related wastes (Deydier et al., 2005). However, this method has ecological disadvantages in terms of energy expenditure. A current value-added use for poultry feathers are their conversion into feather meal, a digestible dietary protein for animal feed produced by treating feathers with wet heat (Morris et al., 1973). Due to a high degree of cross-linking by disulfide bonds, hydrogen-bonding and hydrophobic interactions, keratin is resistant to degradation by common proteolytic enzymes such as trypsin, pepsin and papain (Williams et al., 1990). However, in nature, keratins are hydrolyzed by some microorganisms that synthesize keratinases. These enzymes have the ability to degrade native keratin into smaller peptide entities that can subsequently be absorbed by cells. Keratinolytic enzymes are widespread in nature and are elaborated by several microorganisms; most of them are isolated from poultry wastes. These organisms include bacteria, actinomycetes and fungi (Gupta and Ramani, 2006).
This study is an effort to screen out fungi that are capable of invitro degradation of keratinous substances like bird feathers and animals hair. Biochemically, keratin differs from other proteins in having a higher content of sulfur containing aminoacids. The disulfide bonds between these aminoacids make it resistant to most proteases (Dalev et al., 1994). The primary objective of this study was to determine the types of fungi found on the hair of different domestic animals and birds in Conoor district, Ooty. This would facilitate to know the distribution and occurrence of keratinophilic fungi, which could have a role in degradation of keratinous material as an industrial point of view.
Materials and Methods
The incidence of keratinophilic and keratinolytic fungi in animal hairs and bird feather samples were selected, a total of 97 trunk hair samples from both animals and birds were collected from the slaughter houses in Coonoor located at the Nilgiri district during the month from November to December 2006. The samples were collected from the live animals and birds with the help of sterile forceps and packed in sterile polythene cover to avoid contamination.
Table 1
| S.No | Name of the animal | No. of Samples collected | Total no. of samples collected |
| BIRD FEATHER SAMPLES | |||
| 1 | Hen | 40 |
75
|
| 2 | Wild Chicken ( Kaadai) | 15 | |
| 3 | Turkey | 10 | |
| 4 | Pigeon | 10 | |
| ANIMAL HAIR SAMPLES | |||
| 1 | Rabbit | 5 |
22 |
| 2 | Goat | 10 | |
| 3 | Cow | 5 | |
| 4 | Cat | 1 | |
| 5 | Dog | 1 | |
| TOTAL | 97 | ||
Isolation of keratinophilic fungi
By direct inoculation method
The hair samples were surface sterilized using 90% alcohol, for 2 minutes. Sabouraud’s dextrose agar [SDA-Hi media] was prepared and sterilized for 15 minutes in autoclave at 121°C. At the hand bearable temperature (50°C), the antibiotic streptomycin was added and the media were poured into the petri dishes and left for solidification. The sterile hair samples were inoculated such that, one end of the sample was being inserted into the medium and the other end was on the surface of the medium. The plates were than incubated at room temperature for 2-3 days until the growth occurs. After the appearance of the fungal growth, macroscopic and microscopic examinations were made.
Examination of fungal isolates
Macroscopic Examination
The colony morphology of the grown fungal colonies was observed by the pigment production in both front and reverse side of the plates.
Microscopic Examination
Tease mount preparation
A drop of Lactophenol Cotton Blue stain [LCB] was placed on the non-greasy, sterile glass slide. With the help of the teasing needle a tuft of fungal hyphae/mycelium with the spores were placed on the slide and the strands of the hyphae were teased slowly and gently to avoid disruption of the conidia.
Scotch tape preparation
A piece of scotch tape was wrapped around a tongue blade with the sticky end exposed out. The surface of the fungal colony was touched with the scotch tape’s sticky end and laid it onto the drop of LCB stain placed on the glass slide. The end of the tape was wrapped over the end of the slide without “Scooting” to prevent the disruption of the conidia. Then, the slide was observed by low power 10X and high power 45X objectives.
Hair Bait technique [Ulfig, 2003]
This technique was mainly used to isolate the keratinophilic and keratinolytic fungi which can utilize keratinous substrates. Soil is well known to support the transient or ongoing existence of keratinophilic fungi and potential sources of infection for human and animals. The basic principle of this method was to use the natural keratin substrate as baits to recover these fungi from soil. Soil samples were collected in sealed polythene bags using sterile spatula/spoon. The hair samples were wrapped in a paper and autoclaved for 15 minutes at 121°C.
Hair Baiting
Sterile petri dishes were taken and evenly spread with the soil samples. About 10- 30 pieces of hair samples were placed onto the soil. The soils were sprayed with distilled water and watered regularly in order to avoid drying. The plates were incubated at the room temperature until white to brown fungal colonies appeared. Once the fungal growth appeared, the hair samples were taken with the fungus growth and were inoculated onto the SDA medium and incubated. The colonies were later observed macroscopically and microscopically and identified with the help of standard procedure (Udhaya prakash, 2004; Fisher and Cook, 1988).
Identification of keratinolytic activity
The purpose of investigation of keratinophilic activity was useful for the identification and confirmation of keratinolytic activity of fungi isolated through SDA and HBT methods. Keratinolytic activity was confirmed by the degradation of the hair samples inoculated with isolated fungal cultures. The soil samples used were of two types, one was an ordinary soil sample obtained from garden subsoil and the other was slaughter house waste land soil. The soil samples were spread evenly on the petri plates and the sterile hair samples were placed on them. The hair samples were inoculated with identified fungal isolates. The soil samples were sprayed with sterile distilled water without flooding the plate, so as to wet the surface. The plates were incubated at 25°C for about 2-5 weeks. Hence, the keratinolytic activity was identified by the degradation of hair samples by the inoculated fungal cultures.
Results
A total of 75 bird feathers and 22 animal hair samples (97 samples) were processed for in-vitro degradation of keratin. Both keratinophilic and keratinolytic fungi were identified and among them, 22 fungal genera were isolated which represented about 27 different species. All the 27 isolates were keratinophilic and among them 12 were found to have keratinolytic activity (Table 2 and Figure 1).
Out of the 22 fungal genera isolated, 15 were Keratinophilic, 5 were Keratinolytic and 7 were found to have both kertinophilic and kertinolyitc activity. The prevalence of fungi in Hen’s feather samples revealed that Aspergillus niger (25%) was predominant organism, fungal isolates followed by Fusarium spp. (17.5%) and Trichoderma spp. (10%). The remaining includes Acremonium spp. Alternaria spp. Nigrospora spp. Aspergillus fumingatus were 7.5%. All other isolates such as Aspergillus flavus, A. ochraceous, Aureobasidium spp. Chrysosporium spp. Curvularia spp. were 5% and Cladosporium spp. Scopulariopsis spp. Epicoccum spp. were about 2.5%.
The prevalence of fungi in Kaadai feathers revealed that Aspergillus niger and Scopulariopsis spp. (33.33%) were the most commonest one, Absidia spp. A. terreus, Aureobasidium spp. Exophiala spp. Fusarium spp. Geotrichum spp. Rhizomucor spp. Syncephalastrum spp. and Torulopsis. were around 6.6%.
Scopulariopsis spp.(40%) and Aspergillus niger (30%) were the most commonest isolates were recorded in Turkey feathers followed by Absidia spp. and A. terreus were 20% and other isolates including Acremonium spp. Alternaria spp. A. ochraceous, Aureobasidium spp. Cladosporium spp. Epicoccum spp. and Fusarium spp. were found to be 10%.
Aspergillus niger (60%) followed by Aspergillus flavus (20%) were isolated from pigeon feathers and other organisms including Absidia spp. Acremonium spp. Aspergillus fumigatus, A. glaucus, A. ochraceous, Chrysosporium spp. and Trichoderma spp. were found to be 10%. In Rabbit feathers, Aspergillus niger and Alternaria spp. were the commenst isolates were recorded and Acremonium spp. A. terrnus, Aureobasidium spp. and Curvularia spp. were occurred minimum per cent. Acremonium spp. Alternaria spp. Aspergillus niger. Fusarium spp. and Rhizopus spp. were equally occurred in cow hairs (20%). Aspergillus niger was the predominant fungal isolate (70%) occurred in goat hairs followed by A. flavus, A. glaucus, A. ochraceous and Cladosporium spp. were recorded (10%). In Cat and Dog hair samples, Alternaria spp. Fusarium spp. and Exophiala spp. were recorded equally.
The over all distribution of fungi isolates in animals and birds hair samples revealed that Aspergillus niger was one of the predominant organism (38 isolates) followed by Fusarium spp. (19 isolates) and A. ochraceous (10 isolates). All other organisms were isolated below 10.
Discussion
Keratinophilic fungi play an important role in the natural degradation of keratinized residues (Ali-Shtayeh et al., 2000). Keratins are the most abundant proteins in epithelial cells of vertebrates and represent the major constituents of skin and its appendages such as nail, hair, feather, and wool. The protein chains are packed tightly either in α-helix (α-keratins) or in β-sheet (β-keratins) structures. Keratins are grouped into hard keratins (feather, hair, hoof and nail) and soft keratins (skin and callus) according to sulphur content. (Matikeviciene, 2009).
World-wide poultry processing plants produce millions of tons of feathers as a waste product annually, which consists of approximately 90% keratin. Feathers represent 5-7% of the total weight of mature chickens and they constitute a sizable waste disposal problem. Treatment of the feathers with keratin-degrading microorganism may be a better alternative to dispose the poultry waste and reduce environmental pollution.
Keratinophilic fungi colonize on various keratinous fungi colonize on various keratinous substrates and degrade them to components of low molecular weight [Hubalek, 2000]. Worldwide, poultry-processing plants produce millions of tons of feathers annually as a waste product. Their β-Keratin content is largely responsible for their high degree of recalcitrance in degradation processes. Many Keratinophilic fungi frequently parasitize keratinous tissues like skin, nails and hair in man and animals. Keratinophilic fungi are present in the environment with variable distribution patterns that depend on the different factors such as human and or animal presence which are of fundamentals important [Gugnani, 2000].
In this present investigation, 97 samples of hair were collected from different animals and birds. About 56 isolates were obtained, among which 27 fungi were identified and the remaining 29 were left unidentified. The identified isolates were Absidia spp. Acremonium spp. Auneobasidium spp. Alternaria spp., Aspergillus niger, A. flavus, A. fumingatus, A. ochraceous, A. glaucus, Curvularia spp., Cladosporium spp. Collectotrichum spp. Chrysosporium spp. Epicoccum spp. Exophiala spp. Fusrium spp. Geotrichum spp. Nigrospora spp. Rhizomucor Rhizopus spp. Phoma spp. Scopulariopsis spp. Syncephalastrum spp. Trichoderma spp. Torulopsis spp. and Aspergillus terreus.
The prevalence of keratinophilic fungi was significantly higher in bird feather samples than in the animal hair samples. Increased poultry operation have resulted in the generation of large volumes of chicken waste feathers were do not accumulate in nature (Anil kumar,1995).
In the present study the following isolates were recorded, such as – Alternaria spp. Aspergillus niger, A. glaucus, A. flavus, A. ochraceous, A. fumigatus, A. terreus, Curvularia spp. Chaetomium spp. Chrysosporium spp. Exophiala spp. Fusarium spp. Geotrichum spp. Phoma spp. and Scopulariopsis spp. These are the numerous non-dermatophytic filament Keratinophilic fungi belonging to diverse taxonomic groups. These results were similar to the results of Gugnani (2000).
Chrysosporium spp. Absidia spp. Acremonium spp. Aspergillus niger., Aspergillus terreus, Alternaria spp., Rhizomucor spp., Fusarium spp., Scopulariopsis spp., Syncephalastrum spp., Colletotrichum spp., and Curvularia spp., had produced keratinolytic activity which prelates the result obtained by Ulfig (2003).
Table 2
| S.No | Type of the organism isolated | No of isolates | No of % |
| 1 | Keratinophilic fungi (Kp) | 15 | 55.6 |
| 2 | Keratinolytic fungi (Kl) | 5 | 18.5 |
| 3 | Keratinophilic and Keratinolytic
fungi (Kp and Kl) |
7 | 25.9 |
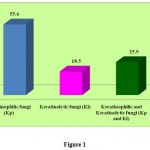 |
Figure 1
|
Table 3: Incidence of fungal flora on Bird feather samples and Animal Hair samples.
| S.No | Fungal Isolates | Type of the isolates (Kp / Kl) | Bird Feather Samples | Total isolates from birds | Animal Hair Samples | Total isolates from animals | Total isolates from birds & animals | |||||||
| Hen n=40 | Wild Chicken n=15 | Turkey Chicken = 10 | Pigeon n=10 | Rabbit n=5 | Cow n=5 | Goat n=10 | Cat n=1 | Dog n =1 | ||||||
| Inc | Inc | Inc | Inc | Inc | Inc | Inc | Inc | Inc | ||||||
| 1 | Absidia spp. | Kl | – | 1 | 2 | 1 | 4 | – | – | – | – | – | 0 | 4 |
| 2 | Acremonium spp. | Kl | 3 | – | 1 | 1 | 5 | 1 | 1 | – | – | – | 2 | 7 |
| 3 | Alternaria spp. | Kp | 3 | – | 1 | – | 4 | 3 | 1 | – | 1 | – | 5 | 9 |
| 4 | Aspergillus flavus | Kp | 2 | – | – | 2 | 4 | – | – | 1 | – | – | 1 | 5 |
| 5 | Aspergillus fumigatus | Kp | 3 | – | – | 1 | 4 | – | – | – | – | – | 0 | 4 |
| 6 | Aspergillus glaucus | Kp | – | – | – | 1 | 1 | – | – | 1 | – | – | 1 | 2 |
| 7 | Aspergillus niger | Kp & Kl | 10 | 5 | 3 | 6 | 24 | 6 | 1 | 7 | – | – | 14 | 38 |
| 8 | Aspergillus ochraceous | Kp | 2 | 4 | 1 | 1 | 8 | – | – | 1 | – | – | 1 | 9 |
| 9 | Aspergillus terreus | Kp & Kl | 2 | 1 | 2 | – | 5 | 1 | – | – | – | – | 1 | 6 |
| 10 | Aureobasidium spp. | Kp | 2 | 1 | 1 | – | 4 | 1 | – | – | – | – | 1 | 5 |
| 11 | Cladosporium spp. | Kp | 1 | 2 | 1 | – | 4 | – | – | 1 | – | – | 1 | 5 |
| 12 | Chryosporium spp. | Kp | 2 | – | – | 1 | 3 | – | – | – | – | – | 0 | 3 |
| 13 | Collectotrichum spp. | Kp & Kl | – | 2 | – | – | 2 | – | – | – | – | – | 0 | 2 |
| 14 | Cunninghamella spp. | Kp & Kl | – | – | – | – | 0 | – | – | 1 | – | – | 1 | 1 |
| 15 | Curvularia spp. | Kp & Kl | 2 | – | – | 1 | 3 | 1 | – | – | – | – | 1 | 4 |
| 16 | Epicoccum spp. | Kp | 1 | – | – | 1 | 2 | – | – | – | – | – | 0 | 2 |
| 17 | Exophiala spp. | Kp | – | 1 | – | – | 1 | – | – | – | – | 1 | 1 | 2 |
| 18 | Fusarium spp. | Kp & Kl | 7 | 1 | 1 | 1 | 10 | 6 | 1 | – | 1 | 1 | 9 | 19 |
| 19 | Geotrichum spp. | Kl | 6 | 1 | – | – | 7 | – | – | – | – | – | 0 | 7 |
| 20 | Nigrospora spp. | Kp | 3 | – | – | – | 3 | – | – | – | – | – | 0 | 3 |
| 21 | Phoma spp. | Kp | 1 | – | – | – | 1 | – | – | – | – | – | 0 | 1 |
| 22 | Rhizopus spp. | Kp | – | – | – | – | 0 | – | 1 | – | – | – | 1 | 1 |
| 23 | Rhizomucor spp. | Kl | – | 1 | – | – | 1 | – | – | – | – | – | 0 | 1 |
| 24 | Scopulariopsis spp. | Kp & Kl | 1 | 5 | 4 | 1 | 11 | – | – | – | – | – | 0 | 11 |
| 25 | Syncephalastrum spp. | Kl | – | 1 | – | – | 1 | – | – | – | – | – | 0 | 1 |
| 26 | Trichoderma spp. | Kp | 4 | – | – | 1 | 5 | – | – | – | – | – | 0 | 5 |
| 27 | Torulopsis spp. | Kp | – | 1 | – | – | 1 | – | – | – | – | – | 0 | 1 |
| TOTAL | 55 | 27 | 17 | 18 | 117 | 19 | 5 | 12 | 2 | 2 | 40 | 157/158 | ||
* Inc. – Incidence, Kp – Keratinophilic fungi, Ki – Keratinolytic fungi
Conclusion
Keratin is one of the most abundant animal proteins on earth as it forms a part of the exoskeleton of reptiles, birds and mammals. Among the microbes that cycle this protein in nature, keratinophilic fungi are very common and the most diverse. During the course of evolution, many of the soil-associated keratinophilic fungi have adopted a pathogenic life cycle and are now potential agents of fungal diseases in humans and animals. If keratinophilic fungi were not there to cycle this highly stable protein (keratin), then one can imagine the quantity of keratin that would have accumulated on earth, since a vast quantity of Keratin is shed by the vertebrates. Indian soils contain many more keratinophilic fungi than those presently recorded and there is need for further taxonomic and ecological studies of this interesting group of organisms.
References
- Ali-Shtayeh, M.S. and Jamous, R.M.J., Keratinophilic fungi and related dermatophytes inpolluted soil and water habitats. Department of Biolgical Sciences, An-Najah Nat. University Nablus, Palestinian University: 51-59 (2000).
- Anil Kumar and Shrivastava, J.N., Keratinophilic fungi: A microbial way to mange poultry waste feathers. Ind.J.of Microbiol vol 45., 2: 151-154 (2005.)
- Asian J Microbiol Biotech Env Sc 4; 251-254. Khanam, S.J.P., Agarwal, S.C. and Jain P.C., Keratinophilic fungi from soils of coal mining areas of Chatisgarh (India). J Basic Appl Mycol., 1: 11-15 (2002).
- Baxter, M. and Trotter, M.D., The effect of fatty materials extruded from keratins on the growth of fungi with particular reference to fatty acid content. Sabouaudia., 7: 199-206 (1969).
- Baxter, M., Ringworm due to Microsporum canis in dogs and cats in New Zealand. New Zealand vet J., 21: 33-37 (1973).
- Dalev, P.G., Utilization of waste feathers from poultry slaughter for production of a protein concentrate. Biores Technol., 48: 265-267 (1994).
- Das, S.K. and Banerjee, A.B., Lipolytic enzymes of Trichophyton rubrum. Sabouraudia., 15: 313- 323 (1977).
- Deshmukh, S.K., Agarwal, S.C. and Jain, P.C., Colonization of fresh and defatted feathers by Keratinophilic fungi. Mykosen., 24: 611-613 (1981).
- Deshmukh., S.K., Mandeel, Q.A., and Verekar, S.A., Keratinophilic fungi from selected soils of Bahrain. Mycopathologia 165: 143-147 (2008).
- Deydier, E., Guilet, R., Sarda, S., and Sharrock, P., Physical and chemical characterization of crude meat and bone meal combustion residue: “Waste or raw material?” J Hazard Mater., 121: 141-148 (2005).
- Ganaie, M. A., Sood, S., Rizvi, G., and Khan, T.A. Isolation and Identification of Keratinophilic fungi from different soil samples in Jhansi City (India). Plant Pathology Journal.Vol.9, No. 4: 194-197 (2010).
- Gugnani, H.C., Non-dermtophytic filamentous kertainophilic fungi and their role in human infection. Department of Med.Mycology.Vallabhai Peterchest Institute. University of Delhi, India, 109-113 (2000).
- Gupta, R. and Ramani, P., Microbial keratinases and their perspective application: an over view. Appl.Microbiol.Biotechnology., 4: 1-13.
- Hubalek, Z., Keratinolytic fungi in wild vertebrates. Institute of Vertebrate Biology, Academy of sciences, Valtice, Czech Rep: 93-103 (2000).
- Irshad Hussain Soomro, Abdul Hussain Shar, and Fateh Mohammad Soomro. Fungal biota of the domestic animals in a city in Pakistan. Pak J Med Sci Vol. 26 No. 4. 964-967 (2010).
- Irshad Hussain Soomro, Yasmeen Faiz Kazi, Miandad Zardari and Abdul Hussain Shar. Isolation of Keratinophilic Fungi from Soil in Khairpur City, Sindh, Pakistan. Bangladesh J Microbiol, Vol 24, No. 1: 79-80 (2007).
- Jain, P.C., Khanam, S.J.P. and Agarwal, S.C., Keratin degrading and enzyme production ability of fungi from soil. Ind.J.of Microbiol Vol 44., 4: 261-264 (2004).
- Jayalakshmi, T., Krishnamoorthy, P., Ramesh kumar, G., and Sivamani, P., Isolation and Screening of a Feather-Degrading Keratinolytic Actinomycetes from Actinomyces spp. Journal of American Science; 6 (12): 45 – 48 (2010).
- Khanam, S.J.P. and Jain, P.C., Isolation of keratin degrading fungi from soil of Damoh (India) (2002).
- Kunert, J., Physiology of keratinophilic fungi. In: Biology of Dermatophytes and other keratinophilic fungi (Kushwaha R.K.S and Guarro J eds). Revista Ibero Americana de Mycologia, Spain., 77-85 (2000).
- Moallaei, H., Zaini, F., Pihet, M., Mahmoudi, M., and Hashemi, J., Isolation of Keratinophilic Fungi from Soil Samples of Forests and Farm Yards. Iranian J Publ. Health, Vol. 35, No. 4: 62-69 (2006).
- Morris, W.C., and Ballown., S.L., Effect of processing methods on utilization of feather meal by broiler chicks. Poultry Science., 52: 858-866 (1973).
- Onifade, A., Al-Sane, N., Al-Musallan, A., Al-Zarban, S., Review : potentials for biotechnological applications of keratin degrading micro-organisms and their enzymes for nutritional improvement of feathers and others, keratins as livestock feed resources. Biores Technol., 66: 1-11 (1998).
- Overview. Appl Microbiol biotechnology., 4: 1-13 (2006).
- Pugh, G.J.F. and Evans, M.D., Keratinophilic fungi associated with birds. II Physiological Studies.Trans Br Mycol Soc., 54: 241-250 (1970).
- Rahul Sharma and Rajak. R C., Keratinophilic Fungi: Nature’s Keratin Degrading Machines! Their Isolation, Identification and Ecological Role. Resonance (2003).
- Santos, R.M.D.B., Firmino, A.A.P., de Sa C.M. and Felix, C.R., Keratinolytic activity of Aspergillus fumigatus Frasemius. Curr Microbiol., 33: 364-370 (1996).
- Snider, R., Landers, S. and Levy, M.L., The ring worm riddle: An outbreak of Microsporum canis in the nursery. Pediatr Infect Dis J., 12: 145-148 (1993).
- Ulfig. K., Studies of Keratinolytic and keratinophilic fungi in sewage study by Means of a Multi – Temperature Hair Bating Method., 12: 461-466 (2003).
- Veslava Matikeviciene, Danute Masiliuniene, and Saulius Grigiskis. Degradation of keratin containing wastes by bacteria with Kertinolytic activity. Environment. Technology. Resources Proceedings of the 7th International Scientific and Practical Conference. Vol 1 (2009).
- Williams, C., Richter, C. Mackenzie, J., Shih and J., Isolation, identification and characterization of a feather degrading bacterium. Appl Environ Microbiol., 56:1509-1515 (2000).
- Williams, C.M., Lee, C.G., Garlich, J. D., and Shih, J.C.H., Evaluation of a bacterial feather fermentation product, feather, lysate as a feed protein. Poult. Sci. 70:85-94 (1991).

This work is licensed under a Creative Commons Attribution 4.0 International License.





