How to Cite | Publication History | PlumX Article Matrix
Meghasham N. Narule
Department of Chemistry, RTM Nagpur University, Nagpur - 440 033 (India).
ABSTRACT: A facile synthesis of 2[2/-propene-1-one-3-(4-hydroxy, 3/-azophenyl) pheny] pyrroles (3a-h) and 2[2/-propene-1-one-3-(4/-hydroxy naphthyl-1-azo) pheny]pyrroles (6a-c) has been achieved by 2[4/-hydroxy benz-1(propane-1-one)] pyrrole 2 and 2[3/-amino benz-1(propene-1-one) ] pyrrole 5 respectively The newly synthesized compounds structures have been supported by IR, 1H NMR, spectral data. The antibacterial and antifungal activities of the compounds have also been evaluated.
KEYWORDS: Pyrrole; azo compounds; 4-hydroxy benzaldehyde
Download this article as:| Copy the following to cite this article: Narule M. N. Synthesis of 2[2/-propen-1/-one,3/-(4-hydroxy,3-azophenyl) Phenyl] Pyrroles and 2[2/-propen-1/-one-3/- (3-hydroxy Naphthyl-1-azo) Phenyl] Pyrroles. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Narule M. N. Synthesis of 2[2/-propen-1/-one,3/-(4-hydroxy,3-azophenyl) Phenyl] Pyrroles and 2[2/-propen-1/-one-3/- (3-hydroxy Naphthyl-1-azo) Phenyl] Pyrroles. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9626 |
Introduction
A broad spectrum of biological activity is associated with both simple and fused pyrrole and a large number of natural and synthetic compounda containing such moieties find pharmaceutical applications1-4. Azo compounds have been found to possess wide spectrum of biodynamic properties. Many of them have been reported as antibacterial5, antimicrobial6, diagnostic aid7, antineoplastic8, urinary antiseptic9 and topical dermatologic activities10. Several azo compounds have been proved useful for the colouration of cellulose acetat fibres.
Result and Discussion
In view of these observations, it was thought worth-while to synthesize and investigate the compounds in which azo group have been linked with pyrrole moiety.
The reaction sequence leading to the formation of desired heterocyclic compounds are outlined in Scheme-I. The starting material 2-[4-hydroxy benz-1(propene-1-one)]Pyrrole (2) was prepared by the reaction of 2-acetyl pyrrole with 4-hydroxy benzaldehyde in presence of 40 % NaOH which on coupling with different aromatic amines in presence of NaNO2 and HCl at 0-50C yielded 2[2/-propene-1-one-3-(4-hydroxy, 3/-azophenyl) phenyl] pyrroles (3a-h) The nitro group present in compound (4) is reduced by Sn/HCl to yield 2[3/-amino benz-1(propene-1-one) ] pyrrole (5) which was coupled with different aromatic hydroxy compounds in presence of NaNO2 and HCl at 0-50C to give 2[2/-propene-1-one-3-(4/-hydroxy naphthyl-1-azo) phenyl]pyrroles (6a-d). The UV-Vis-spectra of the azo dyes (3a-h) and (6a-d) were recorded and the values of absorptions (l max) and fastness properties are shown in Table –I. It is apparent that the wavelength of maximum absorptions azo compound was observed at 200-500nm in EtOH solutions. Variation in lmax is being attributed to structural variation of electron–rich aromatic compounds with N=N linkage used for the preparation of these azo compounds.
Table 1: (UV-VIS Section of Azo compound (3a –h)and (6a-d)and fastness properties.
| Code Colour lmax Fastness properties | ||||||
| Silk | Wool | |||||
| Lighta |
|
Lighta
|
washb
|
Washb | ||
| 3a | Red | 475 | 2 | 3 | 2-3 | 3-4 |
| 3b | Brown | 456 | 3-4 | 2-3 | 3-4 | 2 |
| 3c | Brown | 442 | 2 | 4 | 2 | 3 |
| 3d | Brown | 411 | 2-3 | 3-4 | 2-3 | 2-3 |
| 3e | Orange | 422 | 4 | 2-3 | 3 | 3-4 |
| 3f | Orange | 445 | 2-3 | 3-4 | 2-3 | 2-3 |
| 3g | Red | 470 | 3-4 | 2-3 | 3-4 | 2 |
| 3h | Red | 474 | 2 | 4 | 3 | 2-3 |
| 6a | Red | 473 | 3 | 2-3 | 3-4 | 3 |
| 6b | Orange | 457 | 3-4 | 3 | 2-3 | 2-3 |
| 6c | Orange | 420 | 2 | 3 | 4 | 2-3 |
| 6d | Purple | 483 | 4 | 3-4 | 2-3 | 3 |
IN EtOH solution (3a-h, 6a-d)
aLight-fastness: 1-minimum, 2-poor, 3-moderate, 4-fairly good, 5-good. 6-very good.
bwash-fastness: 1-poor, 2-fair, 3-good, 4-very good and 5-excellent.
Structure proof for the synthesised compounds 3a-h , 6a-d was illusidated by IR and 1HNMR studies. IR spectrum shows the presence of NH-pyrrole group at 3348cm-1, C = 0 group at 16438cm-1, N =N group at 1589cm-1, OH group at 3410cm-1, C-N group at 1587, C–Cl group at 755cm-1, C-NO2 group at 748. 1HNMR spectrum showed be presence 8.1 (s, 1H, NH-pyrrole), 6.8 – 7.0(Ar- H), 5.3 (s, 1H, OH).
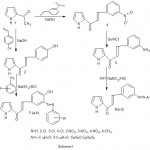 |
Scheme 1
|
Pharmacological activities
Comparative study of the 2 – acetyl pyrrole ( 1 ) and 2 [ 2/ – propene -1-one – 3 – ( 4 – hydroxy , 3 / – azo phenyl ) phenyl ] pyrroles (3 a –h) and 2 [ 2 /– propene – 1 – one –3 –( 4 / – hydroxy naphthyl – 1 – azo ) phenyl ] pyrroles ( 6 a – d ) has been observed by using Norfloxacin and Griseofulvine as standards.They show antibacterial activities against E. Coil and S. Aureus and antifungal activities against A. niger and C. albicans at 100 ug \ ml concentration as shown in Table ( II ).
Table 2: Data for in Vitro antibacterial and anti Fungal activities (in mm)
| Comp | Minimum Inhibitory concentration’s m g /ml | |||
| E. coli | S. aureus | A. niger | C. albicans | |
| 3a | 13 | 15 | 17 | 12 |
| 3b | 15 | 12 | 16 | 18 |
| 3c | 12 | 14 | 15 | 14 |
| 3d | — | 10 | — | 17 |
| 3e | 10 | 12 | 15 | 18 |
| 3f | 14 | 14 | 16 | 17 |
| 3g | NA | 17 | 10 | NA |
| 3h | 9 | 10 | 14 | 17 |
| 6a | 13 | 12 | 17 | 16 |
| 6b | 12 | 9 | 10 | 19 |
| 6c | NA | 14 | 18 | 15 |
| 6d | 14 | 11 | 21 | 14 |
NA – Not active
— = No inhibition of growth
Norfloxcin 100ug/ml used as standard against E. coli, and S. aureus, diameter of zone of inhibition is 20.
Grisefulvin 100ug/ml used as standard against A. niger and C. albicans, diameter of zone of inhibition is 32.
Experimental section
The melting points are uncorrected. Purity of the compounds was checked on silica gel G plates using iodine vapour as visualizing agent. Synthesized compound was characterized by IR spectra, run in KBr on a Perkin – Elmer infrared spectrophotometer. 1H NMR spectra on Brucker AC–300F(300Hz) NMR spectrometer using DMSO-d6 as a solvent and tetramethyl silane as internal standard.
Table 3: Characterization data of newly synthesized compounds 3a-h, 6a-d.
| Comp | R | Mol Formula | M.P.
(°C) |
Yield
(%) |
Analysis formula
(calcd)% (obs) |
||
| C | H | N | |||||
| 3a | – H | C19H14O2N2 | 83 | 65 | 75.2
(75.3) |
4.9
(4.5) |
9.2
(9.1) |
| 3b | 2-Cl | C19H14O2N2Cl | 68 | 92 | 67.2
(67.74) |
4.1
(4.0) |
8.2
(8.3) |
| 3c | 3-Cl | C19H14O2N2Cl | 59 | 61 | 67.2
(67.74) |
4.1
(4.0) |
8.2
(8.3) |
| 3d | 4-Cl | C19H14O2N2Cl | 72 | 58 | 67.2
(67.74) |
4.1
(4.0) |
8.2
(8.3) |
| 3e | 2-NO2 | C19H14O4N3 | 137 | 78 | 65.5
(65.6) |
4.0
(4.2) |
12.0
(12.2) |
| 3f | 3-NO2 | C19H14O4N3 | 149 | 68 | 65.5
(65.6) |
4.0
(4.2) |
12.0
(12.2) |
| 3g | 4-NO2 | C19H14O4N3 | 198 | 68 | 65.5
(65.6) |
4.0
(4.2) |
12.0
(12.2) |
| 3h | 4-CH3 | C20H17O2N2 | 98 | 59 | 75.7
(75.6) |
5.3
(5.1) |
8.8
(8.7) |
| 6a | α-C10H7O | C23H19O2N2 | 163 | 65 | 77.4
(77.4) |
5.3
(5.0) |
7.8
(7.6) |
| 6b | β-C10H7O | C23H19O2N2 | 129 | 62 | 77.4
(77.4) |
5.3
(5.0) |
7.8
(7.6) |
| 6c | -C6H5O | C19H15O2N2 | 122 | 65 | 75.2
(75.3) |
4.9
(4.4) |
7.8
(7.7) |
| 6d | -C6H6O2 | C19H15O3N3 | 185 | 52 | 75.2
(75.6) |
4.5
(4.4) |
12.6
(12.7) |
2[4/-hydroxy benz-1(propane-1-one)] pyrrole 2-acetyl pyrrole (0.01mol) and 4-hydroxy benzaldehyde (0.01mol) was dissolved in 100ml ethanol. To this solution, NaOH (40%, 10ml) was added dropwise with constant stirring at room temp. till a dark yellow mass was obtained.The reaction mixture was kept 7-8 hr and acidified with dil HCl. The solid obtained was washed with cold water. It was filtered and dried. It was crystallized from ethanol. Yield 62% M.P 1530
3a: Preparation of 2[2-propene-1-one-3-(4-hydroxy-3-azophenyl) phenyl ]pyrrole. Aniline (0.1mol)was dissolved in (20ml) 4% HCl and the solution was cooled to 0-5°C. To this saturated sodium nitrite solution was added dropwise followed by addition of compound (2) (0.1mol) in 20ml of 7% NaOH for a period of 10min till the coloured solution is obtained. The solution was stirred for 30min and then neutralized to pH 7 by adding 10% HCl, the solid separated out, filtered dried and crystalized from suitable sovent . Yield 65%:M.P.83°C: IR (KBr) : 3385(-OH), 3130 (NH-pyrrole), 1618 (C= 0), 1520(N=N), 1577cm-1 (C-N), 3144cm-1 (CH of pyrrole); 1H NMR (DMSO-d6); 5.3 (s, 1H, OH), 6.8–8.2(Ar-H), 8.1(d, 1H, NH-pyrrole).
3b:2[2-propene-1-one-3- (4-hydroxy-3-azo-2-chlorophenyl) phenyl]pyrrole.
Yield 92%, M.Pt.68°C; IR (KBr);34229cm-1 (-OH), 3337cm-1 (NH-pyrrole), 1660cm-1 (C = 0), 1545cm-1 (C-N), 3143cm-1 (CH of pyrrole) 1632cm-1 (N=N), 752cm-1 (C-Cl); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 5.3 (s, 1H, OH), 6.3-7.1 (Ar- H).
3c:2[2-propene-1-one-3-(4-hydroxy-3-azo-3-chlorophenyl) phenyl]pyrrole.
Yield 61%, M.Pt.59°C:IR (KBr) ; 34229cm-1 (-OH) 3335, (NH-pyrrole), 1683 (C = 0), 1585cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N) 755cm-1 (C-Cl); 1HNMR (DMSO-d6) 8.7 (1H, s, NH-pyrrole), 6.3 (s, 1H, OH), 7.1 (Ar- H).
3d:2[2-propene-1-one-3- (4-hydroxy-3-azo-4-chlorophenyl) phenyl]pyrrole..
Yield 58% , M.P. 72° C; IR (KBr) ; 34229cm-1 (-OH), 3337 (NH-pyrrole) , 1683 (C = 0), 1547cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N) 742cm-1 (C-Cl) ; 1HNMR (DMSO-d6) 9.2 (1H, s, NH-pyrrole), 5.7 (s, 1H, OH), 6.8 (Ar- H).
3e: 2[2-propene-1-one-3- (4-hydroxy-3-azo-2-nitrophenyl) phenyl]pyrrole.
Yield 78% , M.P. 137° C; IR (KBr) ; 3422cm-1 (-OH), 3335 (NH-pyrrole) , 1683 (C = 0), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N), 742cm-1(C-NO2) ; 1HNMR (DMSO-d6) 8.2 (1H, s, NH-pyrrole), 6.3 (s, 1H, OH), 6.9 (Ar- H).
3f:2[2-propene-1-one-3- (4-hydroxy-3-azo-3-nitrophenyl) phenyl]pyrrole.
Yield 68% , M.P. 149° C; IR (KBr) ; 34229cm-1 (-OH), 3335 (NH-pyrrole) , 1683 (C = 0), 1559cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N) 746cm-1(C-NO2); 1HNMR (DMSO-d6) 8.7 (1H, s, NH-pyrrole), 6.8 (s, 1H, OH), 7.6 (Ar- H).
3g:2[2-propene-1-one-3- (4-hydroxy-3-azo-4-nitrophenyl) phenyl]pyrrole.
Yield 68% , M.P. 198° C; IR (KBr) ; 34229cm-1 (-OH), 3335 (NH-pyrrole) , 1683 (C = 0), 1587cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N) 744cm-1(C-NO2); 1HNMR (DMSO-d6) 9.7 (1H, s, NH-pyrrole), 5.3 (s, 1H, OH), 6.3-7.1 (Ar- H).
3h :2[2-propene-1-one-3- (4-hydroxy-3-azo-4-methylphenyl) phenyl]pyrrole.
Yield 59% , M.P. 98° C; IR (KBr) ; 34229cm-1 (-OH), 3335 (NH-pyrrole) , 1683 (C = 0), 1548cm-1 (C-N), 3044cm-1 (CH of pyrrole) 1635cm-1 (N=N); 1HNMR (DMSO-d6) 9.5 (1H, s, NH-pyrrole), 6.6 (s, 1H, OH), 6.3 (Ar- H).
4: 2[3/-nitro benz-1(propane-1-one)] pyrrole – 2-acetyl pyrrole (0.01mol) and 3-nitro benzaldehyde (0.01mol) was dissolved in 100ml ethanol. To this solution, NaOH (40%, 10ml) was added dropwise with constant stirring at room temp. till a dark yellow mass was obtained .The reaction mixture was kept 7-8 hr and acidified with dil HCl. The solid obtained was washed with cold water. It was filtered and dried. It was crystallized from ethanol. Yield 62 % M.P 1360
6a : Preparation of 2[2-propene-1-one-3-(4-hydroxynaphthyl-1-azo) phenyl] pyrrole.
0.1 mol of compound (5) was dissolved in (20ml) 3% HCl was cooled. The solution to 0-5°C and saturated solution of sodium nitrite was added dropwise maintaining ice cold temperature. The completion of reaction was checked by starch-iodine test. To this solution 0.1gm of a-naphthol in 20ml of 7% NaOH in a period of 10 min. was added. The coloured solution obtained was stirred for 30min. and then nuetralised to pH 7 by adding 10% HCl the solid was separated out, filter dried and crystallized from aqueous ethanol. Yield 68%; M.P. 163°C: IR (KBr) 3330(-OH), 3200(NH-pyrrole), 1672(C= 0), 1630(N=N), 1567cm-1 (C-N), 3010(CH-pyrrole) 1HNMR (DMSO-d6), 9.5 (1H, s, NH-pyrrole), 5.9 (s, 1H, OH), 6.3 (Ar- H).
6b : Preparation of 2[2-propene-1-one-3-(2-hydroxynaphthyl-1-azo)phenyl] pyrrole.
Yield 68%; M.P. 163°C: IR (KBr) 3330(OH), 3200(NH), 1672(C=0), 1630(N=N), 1577cm-1 (C-N), 3122(CH-pyrrole); 1HNMR (DMSO-d6), 8.5 (1H, s, NH-pyrrole), 6.5 (s, 1H, OH), 6.2 (Ar- H).
6c : Preparation of 2[2-propene-1-one-3-(4-hydroxyphenyl-1-azo) phenyl] pyrrole.
Yield 68%; M.P. 163°C: IR (KBr) 3330(OH), 3200(NH), 1672(C= 0), 1630(N=N), 1557cm-1 (C-N), 3011(CH-pyrrole) 1HNMR (DMSO-d6), 9.7 (1H, s, NH-pyrrole), 6.8 (s, 1H, OH), 5.9 (Ar- H).
6d : Preparation of 2[2-propene-1-one-3-(4-hydroxyphenyl-1-azo) phenyl] pyrrole.
Yield 68%; M.P. 163°C: IR (KBr) 3350(OH), 3250(NH), 1670(C= 0), 1631(N=N), 1540cm-1 (C-N), 3020(CH-pyrrole); 1HNMR (DMSO-d6), 9.5 (1H, s, NH-pyrrole), 6.8 (s, 1H, OH), 6.1 (Ar- H).
Acknowledgement
We are thankful to UGC for providing the financial assistance to carry out the research work (F 12-17, 2004, SR). One of the author M.N.Narule is thankful to UGC for research fellowship The authors are also thankful to the Head, Department of Pharmaceutical Science Nagpur University for screening anti-microbial activities, Head RSIC, CDRI, Lucknow for providing the spectral data of the compounds.
References
- F. Battarini, L. Caouzzi, P. Laporta, S. Massimini & Capridiv, Eur pat, (1993), 3276, Chem Abstr, 119, 1993, 49400..
- A. Omar, M. E. Mohsen, W. O. M. Aboul, J.Heterocycl Chem, 21, 1984, 1415..
- F L. Scot, T M. Lambe & R. N. Butter, J. Chem Soc, Perkin Trans, 1, 1972, 269.
- W. Eckhardt, E. Berigar & H. dler, Eur Pat, 371925, Chem Abstr, 1134, 90, 1990, 191385.
- Mietzsch, Klarer, Ger Pat, 638, 1936, 701.
- Gley, Girard, Compt, Rend. Soc. Biol, 125, 1936, 1027.
- Taylor, Thorp, Bit. Heart J, 21, 1959, 492.
- Carter, Friedmabn, Europ, J. Cancer, 8, 1972, 853.
- Dohrten, Diedrich, U. S. Pat,1, 1932, 862, 361.
- Tirasek et. al.,Cersk. Dermatol, 38, 1966, 41.

This work is licensed under a Creative Commons Attribution 4.0 International License.





