How to Cite | Publication History | PlumX Article Matrix
The Effect of Aqueous Leaf Extract of Vernonia amygdalina on Liver Function in Rabbits
E. B. Ezenwanne* and J. Atima Asekhame
Department of Physiology, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City Nigeria.
Correspondences Author E-mail: ezenwanneeeb@yahoo.co.uk
ABSTRACT: A study was carried out to investigate the possible effect of leaf extract of vernonia amygdalina on organic compounds known to be associated with liver function. Twenty female rabbits were placed into four groups of five animals per group. The three test groups received daily oral doses of 400, 200 and 100 mg per kg body weight, of the liquid leaf extract of vernonia amygdalina, respectively, for a period of three weeks. The control group of five animals were placed on normal feeds and received no treatment with vernonia amygdalina extract for the same period. Weekly estimation of the serum concentrations of total bilirubin, conjugated bilirubin and alkaline phosphatase (ALP) revealed significant increase (p £ 0.05) in the serum levels of both total and conjugated bilirubin. The serum alkaline phosphatase level was also increased (p £ 0.05). The results also showed that the observed alteration in conjugated bilirubin was dose-dependent. Sections of liver tissue from the animals obtained at the end of the experiments indicated fibrosis, which appeared to correlate fully with the observed increase in serum bilirubin and alkaline phosphatase levels. The alteration in these parameters is indicative that vernonia amygdalina probably has some clearly definable influence on liver function. The data was also indicative of possible hepatotoxic properties.
KEYWORDS: Vernonia amygdalina; Extract; Effects; Liver function; Rabbit
Download this article as:| Copy the following to cite this article: Ezenwanne E. B, Asekhame J. A. The Effect of Aqueous Leaf Extract of Vernonia amygdalina on Liver Function in Rabbits. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Ezenwanne E. B, Asekhame J. A. The Effect of Aqueous Leaf Extract of Vernonia amygdalina on Liver Function in Rabbits. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9313/ |
Introduction
Vernonia amygdalina is a shrub which belongs to the plant family compositae. The plant is commonly known as bitter leaf in local parlance, and is widely distributed in tropical Africa and Asia. Many communities in Africa hold on to its use as vegetable, a delicacy, it is widely consumed in staple food. In most communities, it is believed that vegetables are the cheapest available source of important vitamins and minerals1.
The roots and leaves of bitter leaf have found wide application in phyto-medicine, and has been shown to be effective in the treatment of a number of illnesses. For example, in Western Uganda it is applied to induce labour, and in the management of retained placenta, post partum bleeding, induced abortion, malaria, bacterial and fungal infections, infertility, colic pain, irregular and painful menstruation2. In Nigeria, the leaves are crushed and rubbed on the breast for weaning infants; bitter leaf is also used extensively for medicinal preparations for the treatment of diabetes and fevers, and the leaf decoction is taken as laxative. Presently, the Nigerian Institute for Pharmacological Research and Development (NIPRD) is set to start the multi-center human clinical trials of a similar drug3.
From cell culture research and animal studies, it was revealed that bitter leaf formula supplement may improve health conditions of immune system of AIDS patients and related symptoms, such as wasting, nausea, vomiting etc. One other very important use of this plant in many countries of Africa lies with its reported application as worm expeller in intestinal parasitic infections4. Not very long ago, it was noted that compounds from the extracts of vernonia amygdalina were able to inhibit the growth of breast cancer, and other tumour cells5. It was also during this same period that some workers listed a number of substances which they claimed were 13 newly discovered chemical compounds in bitter leaf with medicinal properties and therapeutic effects6. Meanwhile, it is now known that vernonia amygdalina is taken in large quantities as a tonic without dose considerations2.
It is obvious from the foregoing account, that vernonia amygdalina is a plant that enjoys a wide range of medicinal and therapeutic uses attributed to it. The shrub is increasingly gaining popularity as vegetable, as well as for its other uses, especially among rural populations throughout Africa and Asia, and even in urban areas in many other parts of the world.
In this discussion, it is important to note that the liver is the largest internal organ in the body, and the fact that it contains diverse enzymes as well as receives large blood supply, places on it a wide range of metabolic functions, including detoxification of foods and drugs, synthesis, secretions, storage of vitamins and iron, excretion etc. Vernonia amygdalina like every other drug consumed orally, passes into the intestine and is absorbed into the blood and then is metabolized in the liver. It was the aim of this study to investigate possible alterations in the serum levels of some of the organic compounds known to be associated with liver function, in doses of aqueous leaf extract of vernonia amygdalina.
Materials and Method
The leaves of vernonia amygdalina obtained from private farms, were sun dried for about 5 days after washing in clean water. Care was taken to ensure that no particles of dust or any dirt remained on the leaves before and during the drying. The carefully dried leaves were crushed into powder in a mortar. The powder was soaked in 1000mls of water for 24 hours to allow for the extraction of constituents. The solution was then filtered and the filtrate placed in an evaporator at 600C for 48 hours. The complete evaporation of water molecules left a sticky and dark greenish extract weighing about 600g. The extract was dissolved in 5000mls of pure water, and the method of earlier workers7 was employed in the preservation, while dose calculations were made based on mg/kg body weight of the animals.
Administration of vernonia amygdalina extract was done orally using an orogastric tube, to ensure that all animals in each test group were given the same amount of extract daily.
Preparation of Animals
Twenty female rabbits of comparable age, size and weight, were procured and allowed to acclimatize in the laboratory for a week. The baseline parameters (including weight, total and conjugated bilirubin, and alkaline phosphatase levels) were measured and recorded. The animals were fed with standard livestock feeds, edible grasses and clean water. The animals were organized in cages into 4 groups of 5 rabbits per group. A group receives one of the doses of the extract for three weeks, as follows:
Group A received 400mg/kg body weight
Group B received 200mg/kg ,, ,,
Group C received 100mg/kg ,, ,,
CONTROL group received no treatment with extract.
At the end of each week, blood was collected from the marginal ear artery, using hypodermic syringes, needles, and micropipettes (1000µl, 100µl). Samples were stored in sample containers and taken to the laboratory for analysis of serum alkaline phosphatase, total and conjugated bilirubin levels using an automated spectrophotometric analyzer.
At the end of the experiments, sections of liver tissues from each of the four groups were fixed in formalin, and were subsequently prepared on slides and observed microscopically for any morphological changes.
Results
The conjugated and total bilirubins revealed significant increase (p ≤ 0.05) in the serum levels, more especially during the 2nd and 3rd weeks of the experimental period, as shown in Tables 1 and 2, respectively. Also the weekly estimated values of the serum alkaline phosphatase levels in the different groups of rabbits showed notable increase, mostly in the 2nd and 3rd weeks of the experimental period compared to the baseline controls (Table 3). The corresponding histopathologic studies of sections of the liver tissues at the end of the experiments, revealed fibrosis which was seen mainly in rabbit groups B and C, as shown in figure 1.
Table 1: The serum conjugated bilirubin (mg/dl) as determined weekly in the different animal groups.
| Baseline | 1st Week | 2nd Week | 3rd Week | |
| GROUP A | 0.20 ±0.04 | 0.40 ±0.04* | 0.45 ±0.05* | 0.50 ±0.03* |
| GROUP B | 0.20 ±0.10 | 0.30 ±0.04 | 0.42 ±0.03* | 0.40 ±0.03* |
| GROUP C | 0.21 ±0.04 | 0.32 ±0.10 | 0.40 ±0.03* | 0.40 ±0.04* |
| CONTROL | 0.20 ±0.10 | 0.22 ±0.10 | 0.20 ±0.04 | 0.21 ±0.04 |
* Significant levels (p ≤ 0.05) are Mean ±SEM compared to baseline values
Table 2: The weekly values of total serum bilirubin (mg/dl) as determined in the various groups of the rabbits.
| Baseline | 1st Week | 2nd Week | 3rd Week | |
| GROUP A | 0.68 ±0.04 | 0.78 ±0.06 | 0.9 ±0.20 | 1.07 ±0.05* |
| GROUP B | 0.64 ±0.02 | 0.72 ±0.03* | 1.3 ±0.20* | 1.40 ±0.04* |
| GROUP C | 0.48 ±0.04 | 0.67 ±0.04* | 0.8 ±0.04* | 0.50 ±0.04* |
| CONTROL | 0.60 ±0.07 | 0.60 ±0.06 | 0.62 ±0.08 | 0.70 ±0.06 |
* Significant levels (p ≤ 0.05) are Mean ±SEM, versus the baseline control
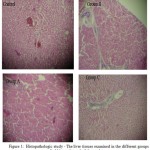 |
Figure 1: Histopathologic study – The liver tissues examined in the different groups of rabbits at the end of the study. |
Discussion
The results of this study indicate clear alterations in both serum bilirubins and alkaline phosphatase enzyme in rabbit. There is also the indication from present data, of possible hepatotoxic effects. The changes in conjugated bilirubin presented in Table 1, revealed that there was clear dose-dependent increase in serum levels, coupled with steady rise in the total bilirubin concentration throughout the experiments (Table 2).
Conjugated bilirubin are those normally transported to the liver bound to albumin. Conjugated bilirubin is lipid soluble, but if not protein bound, bilirubin is able to cross cell membranes, including the blood brain barrier. It has however been noted that at physiological concentration, all bilirubin is protein bound 8. In the liver, bilirubin is known to be transferred from albumin to where it is actively transported to the smooth endoplasmic reticulum, and is conjugated with molecules of glucoronate by a glucronyl transferase and then secreted into the bile canaliculi by active process.
From the data in this study, it can be inferred that the dose-dependent increase in conjugated and total bilirubins was due to the overwhelming effect of excess bilirubin released from hemolysed red blood cells on the conjugating mechanism of the liver. The increase in conjugated bilirubin concentration could also be as a result of obstruction of the hepatobiliary channels which may be due to inflammation of the cells lining the ducts. It was also observed that such inflammation of the cells could be drug or toxin induced9. It is also known that certain drugs may compete for binding, thus, impairing bilirubin conjugation and excretion, and which results in increased total bilirubin.
Enzymes are organic catalysts to biochemical reactions. The assessment determination of certain enzymes in the liver is an important aid in ascertaining the functional state of the liver. In Table 3, serum alkaline phosphatase enzyme concentration showed significant increase (p ≤ 0.05) in the course of the experiments, although this was not in a dose-dependent manner. Increase in alkaline phosphatase is an indicator of cholestasis; obstruction of the bile channels. Increase in alkaline phosphatase could also be extrahepatic since alkaline phosphatase is present in high concentrations in the bone, placenta, intestines etc. However, in this study, the rise in alkaline phosphatase could be of hepatic origin since this is shown to correlate well with the corresponding increase in conjugated bilirubin as noted in Table 1, and the fibrosis observed in the histopathologic studies.
Table 3: Serum alkaline phosphatase levels (iu/l) as determined each week in the rabbits, in various groups.
| Baseline | 1st Week | 2nd Week | 3rd Week | |
| GROUP A | 22.8 ±1.40 | 29.0 ±0.50 | 30.8 ±0.80* | 33.2 ±1.00* |
| PROUP B | 23.4 ±1.10 | 31.1 ±1.30* | 32.5 ±2.10* | 36.1 ±1.10* |
| GROUP C | 22.2 ±1.20 | 26.4 ±1.50 | 33.2 ±0.70* | 36.1 ±1.20* |
| CONTROL | 23.1 ±1.70 | 23.3 ±1.50 | 22.8 ±1.00 | 22.9 ±0.70 |
* Significant levels (p ≤ 0.05) are Mean ±SEM compared to baseline values
Meanwhile, constitutional analysis of vernonia mygdalina have shown that it contains sesquiterpene lactones such as vernodalin, vernodiol and vernomygdin, glycosides like saponin and amygdalin, flavonoids to which its physiologic effects has been attributed8. It had earlier been noted that saponins form unstable interaction molecules with proteins, phospholipids and cholesterol on erythrocyte membranes, resulting in membrane fragility and ultimate hemolysis10.
In this study, results of the histopathologic studies indicated fibrosis, which was observed mostly in the animal groups B and C (Fig. 1). It is possible that ingestion of vernonia amygdalina in high doses and for prolonged periods may result in some toxicity. In other words, it is possible that vernonia amygdalina may possess hepatotoxic properties in large doses. Usually, such very large quantities of the vegetable are not consumed at once. All drugs, and indeed various compounds of plant origin ingested for food are potentially hepatotoxic, because they have to undergo metabolism by the liver. The observed hepatotoxicity could be either directly or by hypersensitivity reactions. However it has been noted that in such cases as hypersensitivity reactions, damage to the liver is usually not dose-dependent6. Similarly, earlier reports have speculated on possible hepatotoxic effects of vernonia amygdalina in rats7.
Since the use of this plant as vegetable in most staple food is becoming increasingly popular, especially amongst rural population in Nigeria and many parts of Africa and Asia, it is expected that further studies may make clearer the specific chemical constituents and properties of vernonia amygdalina, to further elucidate the observed hepatic function effects and, possibly, the mode of action.
Refrences
- Okafor N. and Anichie G. West African hop substitute for sorgum lager. Brewing and distillation international, 13: 20-21 (1983).
- Sade O. Eating bitter leaf prevents diabetes and its complications, native hop news. www.native hop.com/5html (2008).
- WWW.ONLINENIGARIA.COM Daily News .
- Aregheore E., Makkar H., Becker K. Feed value of some browse plants from the cetral. Zone of Delta State, Nigeria.Trop Sci. 38(2): 97-104 (1998).
- Izevbigie E.B. Discovery of water soluble anticancer agents (edodites) from a vegetable found in Benin City, Nigeria. Exp. Biol. Med. 228(3)293-298 (2003).
- Michael A.H. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proceedings of the Nutrition Society. Cambridge University Press. 62: 371-381.
- Ojiako O.A., Nwanjo H.U. Is vernonia amygdalina hepatotoxic or hepatoprotective?. Response from biochemical and toxicity studies in rats. African J. of biotech. 5(18): 1648-1651 (2006).
- Crook M. Clinical chemistry and metabolic medicine. Edward Arnold publishers 7th Ed. 250-268 (2006).
- Erasto P., Grierson D. Afolayan A. Bioactive Sesquiterpene lactones from the leaves of vernonia amygdalina. J. of ethnopharmacology. Elsevier Ireland. 106(1): 117-120 (2006).
- Assa Y., Shany S., Gestetner B., Tencer Y. Birk, Y. Bonbi A., Interaction of alfalfa saponins with components of erythrocyte membrane in hemolysis. Biochimica et Biophysica Acta. 307(1): 83-91 (1973).

This work is licensed under a Creative Commons Attribution 4.0 International License.





