Manuscript accepted on : 12 June 2012
Published online on: --
A Study of Oxidative Stress In Preeclampsia
Chandan K. Nath1, Mriganka Baruah2, Indrajit Nath3, Upasana Baruah4 and Aroon Chandra Bayan5
1Department of Biochemistry, NEIGRIHMS, Shillong, Meghalaya, India.
2Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Science, India.
3Department of Biochemistry, North Bengal Medical College, West Bengal, India.
4Department of O and G, Guwahati, Assam, India.
5Department of O and G, Binapani Nursing Home & Hospital, Barpeta Road, Assam, India.
Corresponding Author E-mail: drmriganka.b@gmail.com
ABSTRACT: Preeclampsia is a pregnancy specific syndrome of reduced organ perfusion secondary to vasospasm and endothelial activation. A number of reports indicate that preeclampsia is associated with elevated blood levels of lipid peroxidation products. The present study was undertaken to evaluate the metabolic correlation between preeclampsia and oxidative stress. 95 no of subjects was selected out for the study out of which normal nonpregnant control group had 25 subjects, normal pregnant control group had 40 subjects & preeclampsia group constituted of 30 subjects. The investigations included are serum lipid profile consisting of serum total cholesterol, serum triglycerides, VLDL, LDL and HDL, serum vitamin E as á- tocopherol and malondialdehyde. There is significant increase in serum triglyceride, serum cholesterol along with LDL and VLDL (p<0.0001) in pregnancy over nonpregnant controls and preeclampsia over pregnant control (p<0.0001). It is observed that in normal controls without pregnancy there is no relationship between serum vitamin E and serum lipid peroxidation as represented by whole blood MDA (malondialdehyde) with a correlation coefficient of 0.13 only. In contrast to this, under the condition of normal pregnancy and preeclampsia the correlation between these two parameters increased with coefficients of correlation (r = - 0.9) with a negative trend indicating a definite and significant degree of inverse relationship between two. It is finally proposed that adequate vitamin E supplementation during pregnancy with proportionate increase in preeclampsia may reduce the consequences of peroxidation induced complications during pregnancy.
KEYWORDS: Preecclampsia; Oxidative Stress; Malondialdehyde
Download this article as:| Copy the following to cite this article: Nath C. K, Baruah M, Nath I, Baruah U, Bayan A. C. A Study of Oxidative Stress In Preeclampsia. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Nath C. K, Baruah M, Nath I, Baruah U, Bayan A. C. A Study of Oxidative Stress In Preeclampsia. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9686 |
Introduction
Preeclampsia is a pregnancy specific syndrome of reduced organ perfusion secondary to vasospasm and endothelial activation (1). It is unique to human pregnancy and is a leading cause of maternal mortality and morbidity and also one of the leading causes of premature delivery and intrauterine growth retardation contributing also to neonatal mortality and morbidity (2).
Pregnancies complicated by preeclampsia begins early in the first trimester in the form of abnormal vascular responses to placentation(3,4). In preeclamptic pregnancies there is failure of second wave of invasion that is trophoblastic invasion into the inner third of the myometrium (3), resulting in a state of arteriolar vasoconstriction, abnormally increased sensitivity to vasopressor peptides and amines, and reduced uteroplacental blood flow (4). More recently, it was shown in normal pregnancy that at 10 to 12 weeks’ gestation, the onset of maternal blood flow in the placenta results in a local increase in oxygen tension and parallel elevation in expression and activity of several antioxidant enzymes (5).The authors hypothesized that a putative diminution of the antioxidant response to this oxygenation stimulus could result in oxidative stress that may lead to trophoblast degeneration and possibly contribute to impairment of trophoblast invasion and diminished remodeling of the spiral arteries(5).A defective response to an oxidant stimulus could therefore be one of the earliest events in preeclampsia. Whatever may be the cause of impaired trophoblast invasion, the resultant inadequacy of placental perfusion is likely to result in oxidative stress. A number of reports indicate that preeclampsia is associated with elevated blood levels of lipid peroxidation products (6). Lipid hydroperoxides function in normal physiology by regulating enzymes and redox-sensitive genes (7). However, uncontrolled lipid peroxidation can result in cellular dysfunction and damage. Many endothelial changes that is endothelial cell damage of potential relevance to preeclampsia can be induced by lipid peroxidation in experimental systems. Study of Stamler in 1959(8) showed that eclampsia-like convulsions and intravascular thrombosis could be demonstrated in term pregnant rats fed on a diet deficient in vitamin E and containing lipid peroxides. Lipid peroxides or oxidized LDL were found to produce increased arterial sensitivity to agonists (9) and inhibited endothelial-dependent vasodilation (10). Falanga, Doni, &Delaini in 1988 demonstrated decreased PGI2 production by increased lipid peroxidation via vitamin E deprivation (11). In addition studies have shown that oxidized LDL or hyperlipidemic sera increased endothelial PGI2 production at 24 hr but inhibited it during longer incubations (48–72 hr) (12), promoted osmofragility and hemolysis of the RBCs and inhibited calcium-ATPase via modification of protein thiols (13). In view of its potentially destructive character, uncontrolled lipid peroxidation has been suggested as an etiologic factor in preeclampsia.
The fatty acid profile in women with preeclampsia may also predispose to oxidative stress. Plasma concentrations of very low density lipoprotein (VLDL) and LDL increase progressively with gestational age as reflected by increases in serum triglycerides and cholesterol (14). Mean plasma triglyceride and free fatty acid concentrations undergo near doubling in women with preeclampsia relative to normal pregnancy (15).
One of the first biomarkers of lipid peroxidation found to be elevated in the plasma of women with preeclampsia was malondialdehyde (MDA), a major metabolite of lipid peroxide breakdown (16). Besides the determination of oxidative damage, many investigations have evaluated antioxidant capacity in the maternal circulation. Vitamin E, the most potent lipid soluble antioxidant, plays an important role in scavenging free radicals and helps in protection of the endothelial membrane in pregnancy. The altered lipid profile in preeclampsia is most likely to be responsible for the variable reports on plasma vitamin E concentrations, because vitamin E is transported by lipoproteins (17).
The decreased antioxidant capacity under the condition of preeclampsia either as a cause or a result is definitely expected to be reflected over the triad of peroxidation target in the form of circulating lipids, vitamin E the antioxidant and malondialdehyde the peroxidized product. Under the interactive situation of vascular involvements leading to increased oxygen tension with its ultimate fate as peroxidative damage of lipid molecules it is expected to be of valuable information in correlating the clinical state of preeclampsia with the alterations in the profile of lipids, vitamin E and malondialdehyde from that of the normal pregnancies. It is therefore proposed in the present investigation estimate the said parameters to prove the relationship between the oxidative stress and scavenging system in preeclamptic syndrome.
Aims and objectives
The present study entitled “A STUDY OF OXIDATIV STRESS IN PREECLAMPSIA” is undertaken with the aim of estimating some of the probe parameters involving
Lipid profile as oxidative target,
Vitamin E as antioxidant and
Malondialdehyde as peroxidized product
And with an objective of evaluating the metabolic correlation between preeclampsia and oxidative stress.
Materials and methods
The study was conducted in The Department of Obstetrics And Gynecology, Gauhati Medical College, Gauhati. The study group consisted of 25 normal controls consisting of normal normotensive nonpregnant women in reproductive age group, 40 normal pregnant controls consisting of pregnant normotensive women in their third trimester, 30 experimental groups consisting of women with preeclampsia in third trimester. Preeclampsia was defined as accepted by the International Society for the Study of Hypertension (Davey & MacGillvray 1998). Patients With History Of Chronic Hypertension, Vitamin E Supplementation, Diabetes Mellitus And Other Medical Disorders. Serum cholesterol, HDL, triglyceride, VLDL and LDL was estimated by the autoanalyser. Serum tocopherol was estimated by the modification of the method of Baker and Frank (1968) based on the principle of reduction of ferric to ferrous ion by vitamin E forming a red complex with α, ά-dipyridyl with maximum absorbance at 520nm. Whole blood malondialdehyde is estimated by the method of Ohkawa et al (18) that is lipid peroxides are converted to malondialdehyde which react with thiobarbituric acid to produce a chromogen giving maximum absorbance at 530nm and is expressed as thiobarbituric acid reactive substance (MDA) in blood.
Results and Discussion
The observation under the present setup of study suggests that preeclampsia is relatively more prevalent among younger age group with initial parity which is also in concertation with other studies (19).
The mean fasting serum triglyceride is increased with very high significance (p<0.0001) in both the pregnant groups with or without preeclampsia than the normal nonpregnant controls. The mean serum triglyceride is observed to be significantly different between both the pregnant groups with higher mean under condition of preeclampsia. There is highly significant differences (p<0.0001) in the mean serum cholesterol levels among the three groups as shown in Table1.
Table 1: Comparision of Mean and Probability.
| # Parameters | Mean ± SD | P Value | ||||
| Normal non-pregnant control(NC) | Normal pregnant Control (NP) | Pre-ecampsia (P) | NC v/s NP | NC v/s P | NP v/s P | |
| Age | 27.08± 2.58 | 26.12±4.64 | 25.13±4.50 | p>>0.05
NS |
P<0.05>0.01
significant |
p>>0.05
NS |
| Triglyceride | 60.92± 15.6 | 228.37±31.6 | 288.73±74.30 | P<0.0001
significant |
P<0.0001
significant |
P<0.0001
significant |
| Cholesterol | 97.68±15.53 | 200 ±20.33 | 275.46±40.99 | P<0.0001
significant |
P<0.0001
significant |
P<0.0001 significant |
| HDL | 40.04±7.11 | 37.45±6.96 | 32.6±4.17 | p>0.05
NS |
P<0.0001
significant |
P<0.0001
significant |
| LDL | 45.456±17.23 | 116.9±8.33 | 185.12±23.84 | P<0.0001
significant |
P<0.0001
significant |
P<0.0001 significant |
| VLDL | 12.18±3.12 | 45.67±6.32 | 57.74±14.86 | P<0.0001
significant |
P<0.0001 significant | P<0.0001
significant |
| Vitamin E | 22.12±4.22 | 15.19±6.72 | 12.91±4.59 | P<0.0001
significant |
P<0.0001
significant |
P<0.05
significant |
| Malondialdehyde | 45.06±13.98 | 60.35±18.14 | 57.15±12.47 | P<0.0001
significant |
P<0.0001
significant |
P<0.05
significant |
Legend #Values are given as mean ± S.D. * NS-Not significant
Table 2: Correlation coefficients of 3 groups
| Correlation group | Correlation Cofficient(R)
|
||
| Normal Nonpregnant controls | Normal pregnant Controls | Preeclampsia | |
| Vitamin E &MDA | 0.13 | 0.94 | 0.9 |
| Triglyceride &MDA | 0.12 | 0.91 | 0.9 |
| Cholesterol & MDA | 0.32 | 0.97 | 0.96 |
At this stage of discussion it may be opined from the interpreted results and observations on triglyceride and cholesterol that under condition of normal pregnancy and preeclampsia both serum triglyceride and serum cholesterol responded with significant increase and the magnitude is higher in preeclampsia than in normal pregnancy
There is highly significant difference in the mean values of HDL between normal pregnancy and preeclampsia signifying absence of significant effect over HDL metabolism under metabolic status of normal pregnancy but there is definite and highly significant effect over HDL metabolism under influence of metabolic state of preeclampsia.
The fetal metabolism is basically dependent on glucose and amino acids as sources for energy and building block (20). Fetal metabolism cannot utilize triglyceride and long chain fatty acids as energy source and following the principle of optimization in any type of metabolic regulation, the fetal metabolic pool is not loaded with or rather kept free of these molecules by impermeability of triacyglycerols and long chain fatty acids exerted by the placental barrier. As a consequence of this phenomenon the fetus is supplied entirely by the maternal glucose and amino acids only and so the mother is imparted with an additional load of these molecules compelling her to maintain relatively higher blood levels of these micro molecules than non pregnant metabolic condition. Three major molecules produced by placenta for this purpose is the hormonal placental lactogen, estrogen and progesterone.
Placental lactogen directly stimulates adipose tissue lipolysis by activating hormone sensitive lipase resulting in increased serum fatty acids. The other two placental steroids i.e. estrogen and progesterone induces insulin resistance by suppressing the insulin inhibition of hormone sensitive lipase resulting with increase in serum triglyceride. This estrogen progesterone axis simultaneously depresses lipolysis through reducing the lipoprotein lipase activity which is insulin dependent ultimately producing a metabolic state with increased triglyceride concentration in the circulation. The overall outcome of these pregnancy related hormonal control of placental origin on lipid and carbohydrate metabolism is that glucose and amino acids are rapidly shunted to the fetal side of the placenta and to compensate the relative lack of glucose in maternal side higher amount of triglyceride is maintained in maternal circulation for longer period by controlling the activity of these two triglyceride regulating enzymes .i.e. lipoprotein lipase and hormone sensitive lipase.The behavior of lipid fractions in preeclampsia definitely suggest either an imbalance of this placental hormonal triad from that of normal pregnancy or presence of some additional modulator produced by placenta or any other source related with preeclamptic condition.
In the present study the mean serum vitamin E are significantly lower (p<0.0001) in normal pregnancy (15.19mg/L) and preeclampsia (12.91mg/L) than the normal non pregnant control (22.12mg/L). There is also significant difference (p<0.05) in the mean serum vitamin E concentration between preeclampsia and normal pregnant controls where the serum vitamin E in preeclampsia is lower than normal pregnancy. The primary role of vitamin E is to act as a biological antioxidant preventing peroxidation of unsaturated and mainly polyunsaturated esterified fatty acids present either in circulation in the form of lipoproteins or in biological membranes formed by phospholipid bilayers. The basic mechanism of action of vitamin E as antioxidant is to act as an electron sink to stray free radicals that escape scavenging by the primary scavengers formed by the triad of superoxide dismutase, glutathione peroxidase and catalase.
Pregnancy is a physiological condition where there is multiple scope for physiological increase in oxygen species generated free radical stress from different sources such as increases in the thyroid function (21) oxidative phosphorylation in the electron transport chain to meet the higher metabolic inducing increased BMR and increased nucleotide biosynthesis to meet the additional demand on hematopoetic system to mention a few sources of enhanced physiological generation of free radicals(22). Under the free radical status of pregnancy it is the most logical assumption that the vitamin E requirement during pregnancy is definitely increased and as the tocopherol molecule sacrifices itself to save the integrity of the membrane lipids a steady dietary input is necessary to maintain a steady circulating vitamin E status. Over and above unlike the other fat soluble vitamins there is no any effective storage system(23) for tocopherol. It is very interesting to observe under the same experimental setup that the serum vitamin E distribution in normal non pregnant controls and in preeclampsia is more consistent or uniform signifying that in normal non pregnant controls the condition is relatively stable due to absence of pregnancy induced vitamin E effectors and in preeclampsia the serum vitamin E status is consistently and uniformly maintained in a lower level even with identical dietary input with the other two groups due to preeclampsia induced enhanced free radical generation .
In the present study malondialdehyde representing as an index for the damage over the unsaturated lipids caused by free radical attack is found to be significantly increased in its mean blood concentration in normal pregnancy (60.35 nmol/ml) preeclampsia (57.15nmol/ml) than the normal non pregnant control group 45.06nmol/ml). The increase in the mean concentration of malondialdehyde is more pronounced in normal pregnancy. It may be noted that malondialdehyde is not a metabolite and its presence in blood only reflects the amount of peroxidized substances the major fraction of which is controlled by lipid and protein peroxidation. So thereby it acts as a reliable index for peroxidation of biological molecules.
From Table 2, It is observed that in normal controls without pregnancy there is no relationship between serum vitamin E and serum lipid peroxidation as represented by whole blood MDA (malondialdehyde) with a correlation coefficient of 0.13 only. In contrast to this under the condition of normal pregnancy and preeclampsia the correlation between these two parameters increased with coefficients of correlation (r=-0.9) with a negative trend indicating a definite and significant degree of inverse relationship between two. This may be explained in the terms that under situation of higher oxidative stress the available vitamin E as antioxidant is utilized and thereby produces a relative vitamin E deficiency status which contributes in peroxidation of more lipids detected as increased MDA. The normal pregnancy trend line is located slightly above in a higher location than preeclampsia due to relatively higher MDA. This may be a consequence of additional peroxidation of some protein or aberrant lipid fraction in preeclampsia which is absent in normal pregnancy.
In the present investigation the relationship between serum triglyceride and whole blood MDA level signifies very high degree of correlation (r=-0.9) between these two parameters in preeclampsia and normal pregnancy which is totally absent in normal non pregnant controls. This indicates that higher triglyceride in presence of relatively high oxidative stress under the two condition makes the lipid more susceptible for peroxidation. Similar relationship is observed between serum cholesterol and whole blood MDA. The similarity in correlation among triglyceride, cholesterol and MDA suggest that under higher oxidative stress and lower vitamin E level lipids such as triglyceride and cholesterol present in the circulating lipoproteins become more susceptible for peroxidation in pregnancy and preeclampsia the effect being more pronounced in preeclampsia. Susceptibility of lipids for peroxidation during pregnancy with further enhancement in preeclampsia appears to be directly proportional to degree of oxidative stress from the observed set of correlation analysis because in any of the combination of these sets the correlation coefficients are very low in the non pregnant control group suggesting minimal relationship between lipid peroxidation and vitamin E under normal level of free radical status.
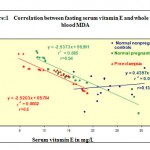 |
Figure 1
|
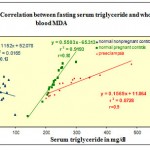 |
Figure 2
|
 |
Figure 3
|
Conclusion
On a comprehensive review of the forgoing discussion it finally emerges out that the physiological alteration of lipid profile during normal pregnancy is enhanced in preeclampsia . The increase in constituents of circulating lipids under both the condition of normal pregnancy and preeclampsia makes them more vulnerable for peroxidation which is more pronounced in preeclamptic condition. The augmented peroxidation in preeclampsia with reference to normal pregnancy may be due to peroxidation of some non lipid molecules associated with preeclamptic metabolism. The vitamin E is observed to have a definite role in preventing or minimizing lipid peroxidation under condition of normal pregnancy and preeclampsia with a threshold limitation with proportion to the free radical induced oxidative load. Above certain threshold proportion overload with oxidative stress depletes available vitamin E under condition of pregnancy which is more pronounced in preeclampsia. As an outcome within the scope and limitation of the present study it may be proposed that adequate vitamin E supplementation during pregnancy with proportionate increase in preeclampsia may reduce the consequences of peroxidation induced complications during pregnancy.
Reference
- Garry Cuningham et al “Williams Obstetrics”, 21st edition ,Singapore.ISBN 0-07-123310-5;P569
- Friedman SA:Hypertension in pregnancy . Curr Opini Obstet Gynaecol ,5:40-49(1993).
- Brosens IA: Morphological changes in the uteroplacental bed in pregnancy hypertension.Clin Obstet Gynecol,4:583-593(1977).
- Khong TY, De Wolf F, Robertson WB. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small for gestational age infants ;Am J Obstet Gynaecol ,93:1049-10595(1986).
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol, 157: 2111–2122(2000).
- Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, Mclaughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol, 161: 1025–1034(1989).
- Smith WL, Marnett LJ, DeWitt DL. Prostaglandin and thromboxane biosynthesis. Pharmacol Ther, 49:153–179(1991).
- Stamler FW. Fatal eclamptic disease of pregnant rats fed anti-vitamin E stress diet. Am J Pathol, 35:1207–1231, (1959).
- Auge N, Fitoussi G, Bascands JL, Pieraggi MT, Junquero D, Valet P, Girolami JP, Salvayre R, Negre-Salvayre A. Mildly oxidized LDL evokes a sustained Ca2+-dependent retraction of vascular smooth muscle cells. Circ Res ,79:871–880(1996).
- Galle J, Ochslen M, Schollmeyer P, Wanner C. Oxidized lipoproteins inhibit endothelium-dependent vasodilation. Hypertension, 23:556–564 (1994).
- Falanga A, Doni MG, Delaini F. Unbalanced control of TxA2 and PGI2 synthesis in vitamin E-deficient rats. Am J Physiol , 245:H867–H870 (1983).
- Myers DE, Huang WN, Larkins RG. Lipoprotein-induced prostacyclin production in endothelial cells and effects of lipoprotein modification. Am J Physiol, 271:C1504–C1511(1996).
- Kagan VE. Lipid Peroxidation in Biomembranes. Boca Raton, FL: CRC Press, Inc., pp55–117 (1988).
- Lorentzen B, Drevon CA, Endressen MJ, Henriksen T. Fatty acid pattern of esterfied and free fatty acids in sera of women with normal and preeclamptic pregnancy. Br J Obstet Gynaecol, 102:530–537 (1995).
- Knopp RH, Bonet B, Lasuncion MA, Montelongo A, Herrera E. Lipoprotein metabolism in pregnancy. In: Herrera E, Knopp R, Eds. Perinatal Biochemistry. Boca Raton, FL: CRC Press, Inc., pp20–51, 1992.
- Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: Current knowledge and future research. Am J Clin Nutr,76: 703–716(2002).
- Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, Mclaughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol, 161: 1025–1034(1989).
- Ohkawa M, Oshishi N & Yagi: Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Analyt. Biochem, 95:357-358(1979).
- Sarkar CS, Giri AK, Sarkar B, Outcome of teenage pregnancy and labour: a retrospective study. J Indian Med Assoc., 89(7):197-9(1991)
- Thomas M Devlin, “Text book of biochemistry with clinical correlations” 2nd edition, John Wiley and Sons, New York. ISBN 0471-83823-3;p 369-374&550.
- Brent GA. Maternal thyroid function: Interpretation of thyroid function tests in pregnancy. Clin Obstet Gynecol,40:3-15(1997).
- Hytten FE, Leitch I: The Physiology of Human Pregnancy, 2nd ed, Philadelphia, Davis, 1971.
- Traber, M.G., 1999. Vitamin E. In: Modern Nutrition in Health and Disease. Ninth Edition. Edited by Maurice Shils, James Olson, Moshe Shike, and A. Catharine Ross. Baltimore: Williams & Wilkins, p. 347-362(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.





