Manuscript accepted on : 29 March 2012
Published online on: --
Antibacterial activity of the Leaf, Stem and Root Powders of Gmelina asiatica L.
A. Shibu1*, S. M. Sundara Pandian2 and S. Dhanam3
1Department of Plant Biology and Bio-Technology, Government Thirumagal Mills College,Gudiyattam.
2Department of Ecology and Environmental Science, Pondicherry University, Puducherry, India.
3Department of Botany, Arignar Anna Arts College, Villupuram, India.
ABSTRACT: Plant are the basic source of knowledge of modern medicine. India is a varietal emporium of medicinal plants and is one of the richest countries in the world in regard to genetic resources of medicinal plants. The present study deals with the Antibacterial activity of Gmelina asiatica L. The stem, root, leaf are powdered and their extracts have been taken from Acetone, Benzene, Chloroform. It is found that the plant extracts have Antibacterial activity against the following nine microbes. Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, Pesudomonas aeruginosa, Proteus mirabilis, Escherichia coli.
KEYWORDS: Antibacterial activity; Gmelina asiatica L.; modern medicine
Download this article as:| Copy the following to cite this article: Shibu A, Pandian S. M. S, Dhanam S. Antibacterial activity of the Leaf, Stem and Root Powders of Gmelina asiatica L.. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Shibu A, Pandian S. M. S, Dhanam S. Antibacterial activity of the Leaf, Stem and Root Powders of Gmelina asiatica L.. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9636 |
Introduction
Ayurveda is a traditional system of medicine using a wide range of modalities to create health and well being. The primary aim of Ayurveda health care is to restore the physical mental and emotional balance in patients, thereby improving health, preventing disease and also treating any current illness. Herbal medicine is still the mainstay of about 75-80% of the world population, mainly in the developing countries for primary healthcare. However among the estimated 250, 000-400,000 plant species, only 6% have been studied for biological activity, and about 15% have been investigated phytochemically. Therefore it seems necessary to evaluate the herbs properly (vandana, et al., 2008).
Infectious diseases are the leading cause of death world-wide. Antibiotic resistance has become a global concern (Westh, et al., 2004). The increasing failure of chemotherapeutics and antibiotic resistance exhibited by pathogenic microbial infectious agents has led to the screening of several medicinal plants for their potential antimicrobial activity (Colombo, et al., 1996; Parekh and Chanda, 2007).
The plant is claimed to be useful in rheumatism, and also possess anti-inflammatory effect. The crude drug of Gmelina asiatica may exert anti-inflammatory activity by anti-proliferative, anti-oxidative and lysosomal membrane stabilization (Ismal, et al., 1997). A bioguided extraction and fractionation of the alcoholic extract of the roots of Gmelina asiatica afforded a new flavones derivative named ovalifolin [3-(3-methyl-1-butenyl)-6-methoxy-5,7,4’-trihydroxy flavones] (+) pinoresinol (Satyanarayana, et al., 2007).
Materials and methods
The present investigation is intented to evaluate the preliminary phytochemical characters such as determination of pharmacogonostic and fluorescence characters, screening of bioactive principles and antibacterial activity of a important medicinal plant, Gmelina asiatica L., Verbenaceae.
Plant description and medicinal use
Gmelina asiatica Linn (Syn. Gmelina parvifolia) is commonly known as “Nilakkumil” in Tamil and “Gopabhadra” in Sanskrit. A large straggling or scrambling deciduous bush or shrub, 3 m tall, petioles 0.5-3 cm long, slender; thin, smooth, leaves small, much-branched leaves small, petioles 0.5-3 cm long, slender, flowers large, yellow colour, calyx about 4 mm long, apically,corolla large, yellow 4-5 cm long, bilabiate,the tube narrow and curvate below4-lobed,fertile stamens 2,staminodes 2(Dassanayake, 1983).
Collection of materials
Material was collected at Perungudi village in Madurai district.The identification was carried out with the help of Flora of the Presidency of Madras.
Preparation of plant extracts
The plant materials were washed with water to remove the adhering dust particles and were shade dried at room – temperature.Extracts were prepared from shade-dried samples following the method of Audu et al. (2000). The dried plant materials were ground into a fine powder in an electric blender. Thereafter 5 g each of fine powdered sample was
weighed and soaked separately in 15 ml of different solvents (Absolute alcohol, Benzene, Chloroform, Ether, and Water) in the ratio of 1:3 weights for volume (W/V). These were
allowed to stand for 24 hrs at ambient room temperature. The soaked plant powder was filtered through filter paper (Whatman No.1) and the filtrate was used as crude extract.
Antibacterial assay and Collection of microorganisms
The microbes were Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Micrococcus luteus, Proteus vulgaris, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcous faecalis . They were obtained from research laboratory, Department of Microbiology, Thiagarajar College, Madurai, Tamil Nadu, India.
Preparation of inoculum
Each organism was recovered for testing by sub-culturing on fresh media. A loopful inoculum of each bacterium was suspended in 5 ml of nutrient broth and incubated overnight at 37oC.
Preparation of media
Nutrient agar is composed of
Beef extract – 3.0 g
Peptone -5.0 g
Agar -15.0 g
Distilled water – 1000 ml
The medium was adjusted to pH 7.4 and sterilized by autoclaving at 15 lbs pressure (121oC) for 15 minutes.
Results and Discussion
Acetone extract of the leaf of Gmelina asiataica showed a well profound, inhibitory activity against Streptococcus pneumoniae, Klebsiella pneumoniae and Micrococcus lateus while stem extract showed a maximum inhibitory activity against Proteus mirabilis and Klebsiella pneumonia (Fig 2a & Fig. A). However, acetone extract of root powder showed a maximum inhibitory activity against Staphylococcus aureus, Bacillus subtilis and
Micrococcus luteus. In general, root extract showed significantly greater inhibitory activity compared to other parts against Staphylococcus aureus.
Table 1: Antibacterial activity of Gmelina asiatica L. against pathogenic bacteria.
| Inhibitory Zone(cm) | ||||||||||||||||||
| Microbes | Leaf | Stem | Root | |||||||||||||||
| A | B | C | E | Et | Aq | A | B | C | E | Et | Aq | A | B | C | E | Et | Aq | |
| S.pneumoniae | 1.2 | 0.8 | 1.25 | 1.25 | 1.19 | 0.8 | 0.7 | 0.2 | 0.9 | 1.1 | 0.61 | 1.8 | 0.4 | 0.43 | 0.5 | 0.39 | 1.2 | 0.5 |
| P.mirabillis | 1.0 | 0.9 | 1.2 | 1.21 | 0.9 | 0.77 | 1.0 | 0.8 | 0.6 | 0.98 | 1.2 | 0.98 | 1.0 | 0.48 | 0.6 | 1.26 | 0.58 | 1.23 |
| K.pneumoniae | 1.2 | 1.0 | 1.1 | 1.42 | 1.3 | 1.21 | 0.9 | 0.72 | 1.2 | 0.39 | 1.1 | 0.78 | 0.9 | 1.1 | 1.3 | 1.24 | 1.21 | 1.22 |
| M.luteus | 1.2 | 0.9 | 1.4 | 1.0 | 1.44 | 1.1 | 0.9 | 0.8 | 1.0 | 1.25 | 0.6 | 1.0 | 0.92 | 0.4 | 1.2 | 1.32 | 1.0 | 1.23 |
| E.coli | 1.0 | 0.91 | 1.2 | 0.8 | 1.2 | 1.22 | 0.4 | 0.3 | 0.98 | 0.82 | 1.1 | 0.98 | 0.12 | 0.42 | 0.7 | 0.6 | 0.5 | 0.3 |
| S.aureus | 0.7 | 0.5 | 1.0 | 1.2 | 0.52 | 1.2 | 0.8 | 0.4 | 0.8 | 0.57 | 0.62 | 0.39 | 2.0 | 1.0 | 0.92 | 0.8 | 0.6 | 0.62 |
| B.subtilis | 1.0 | 1.1 | 0.8 | 0.76 | 1.3 | 0.9 | 0.9 | 0.81 | 1.1 | 1.1 | 1.2 | 0.62 | 1.2 | 1.1 | 1.0 | 1.0 | 1.23 | 1.3 |
| P.aeraginosa | 0.9 | 0.6 | 1.1 | 0.76 | 1.2 | 1.9 | 0.2 | 0.43 | 0.7 | 0.57 | 0.5 | 0.5 | 0.41 | 0.3 | 0.6 | 0.58 | 0.9 | 0.5 |
A-Acetone B-Benzene C-Chloroform E-Ethanol Et-Ether Aq-Aqueous
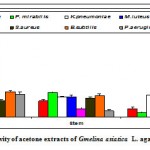 |
Figure 2a: Antibacterial activity of acetone extracts of Gmelina asiatica L. against pathogenic bacteria.
|
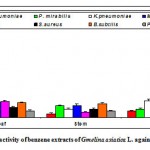 |
Figure 2b: Antibacterial activity of benzene extracts of Gmelina asiatica L. against pathogenic bacteria.
|
| Figure 2c: Antibacterial activity of chloroform extracts of Gmelina asiatica L. against pathogenic bacteria.
Click here to View figure |
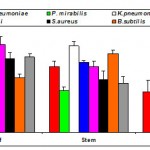 |
Figure 2d: Antibacterial activity of ethanol extracts of Gmelina asiatica L. against pathogenic bacteria.
|
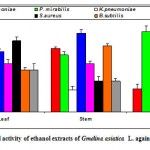 |
Figure 2e: Antibacterial activity of ether extracts of Gmelina asiatica L.against pathogenic bacteria.
|
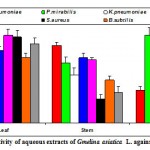 |
Figure 2f: Antibacterial activity of aqueous extracts of Gmelina asiatica L. against pathogenic bacteria.
|
Benzene extract of the leaf of Gmelina asiatica showed a well profound inhibitory activity against Klebsiella pneumoniae, Micrococcus luteus and Escherichia coli, while stem extract showed a maximum inhibitory activity against Bacillus subtilis, Micrococcus luteus and Proteus mirabilis (Fig 2b & Fig. B). However, benzene extract of root powder showed a maximum inhibitory activity against Klebsiella pneumoniae and Staphylococcus aureus. Benzene extract of root powder showed significantly greater inhibitory action than that of other plant parts.
Chloroform extract of the leaf of Gmelina asiatica showed a well profound inhibitory activity against Micrococcus luteus, Streptococcus pneumoniae and Proteus mirabilis, while stem extract showed a maximum inhibitory activity against Klebsiella pneumonia and Bacillus subtilis (Fig 2c & Fig. C). However, chloroform extract of root powder showed a maximum inhibitory activity against Klebsiella pneumoniae, Micrococcus luteus and Bacillus subtilis.
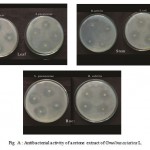 |
Figure A : Antibacterial activity of acetone extract of Gmelina asiatica L.
|
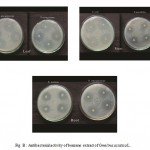 |
Figure B : Antibacterial activity of benzene extract of Gmelina asiatica L.
|
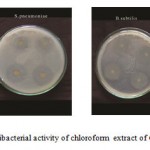 |
Figure C : Antibacterial activity of chloroform extract of Gmelina asiatica L.
|
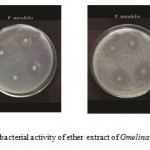 |
Figure D : Antibacterial activity of ethanol extract of Gmelina asiatica L.
|
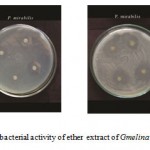 |
Figure E: Antibacterial activity of ether extract of Gmelina asiatica L.
|
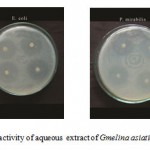 |
Figure F : Antibacterial activity of aqueous extract of Gmelina asiatica L.
|
Ethanol extract of leaf of Gmelina asiatica showed a well profound inhibitory activity against Klebsiella pneumoniae, Streptococcus pneumoniae and Proteus mirabilis, while stem extract showed a maximum inhibitory activity against Micrococcus luteus, Bacillus subtilis and Streptococcus pneumoniae (Fig 2d & Fig. D). Similarly, ethanol extract of root powder showed a maximum inhibitory activity against Micrococcus luteus followed by Proteus mirabilis and Klebsiella pneumonia.
Ether extract of the leaf of Gmelina asiatica showed a well profound inhibitory activity against Micrococcus luteus, Bacillus subtilis and Pseudomonas aeruginosa, while stem extract showed a maximum inhibitory activity against Proteus mirabilis followed by Bacillus subtilis and Escherichia coli (Fig 2e & Fig. E). Similarly, ether extract root powder showed a maximum inhibitory activity against Bacillus subtilis, Klebsiella pneumoniae and Streptococcus pneumoniae.
Aquous extract of the leaf of Gmelina asiatica showed a well profound inhibitory activity against Escherichia coli followed by klebsiella pneumoniae, Staphylococcus aureus and Pseudomonas aeruginosa, while stem extract showed a maximum inhibitory activity against Streptococcus pneumoniae (Fig 2f & Fig. F). However, aqueous extract of root powder showed a maximum inhibitory activity against Proteus mirabilis and Micrococcus luteus.
Conclusion
In the present plant, ”Gmelina asiatica.L” of Verbenaceae family, the powder extract of leaf, stem and root have been taken. In that, the inhibitory activity of the extracts against different bacteria have been noted and tabulated. Of which, the highest inhibitory activity is seen in ethanol and ether extracts of leaf against the two bacteriae (i) Klebsilla Pneumoniae (ii) Micrococcus luteus.
Acknowledgement
I am immensely thankful to Dr. SM.Sundara Pandian for his perfect guidance towards my work. And also I thank my family members for being a good support.
References
- Agaoglu, S., Dostbil, N. and Alemdar, S., Antimicrobial activity of some splces used in the meat industry. Bull vet Inst pulary, 2007; (51) : 53-57.
- Babayi, H., Kolo, I., Okogun, I. and Ijah, V.J.J.,The antibacterial activities of methanolic extracts of Eucalyptus camaldulensis and Terminalia catappa against some pathogenic microorganisms. Journal of Biochemistry, 2004;16: 106-111.
- Coopoosamy, R.M. and Magwa, M.L.,Antibacterial activity of aloe emodin and aloin A isolated from Aloe excelsa. African Journal of Biotechnology,2006;5: 1092 – 1094.
- De Lima, M.R., Souza Luna, J., Santos, A.F., Andrade, M.C., Sant Ana, A.E., Genet, J.P., Marquez, B., Neuville, L. and Moreau, N., Antibacterial activity of some Brazilian medicinal plants. Journal of Ethnopharmacology, 2006;105:137-147.
- Ismail, T.S., Gopalakrishnan, S. and Begum, V.H., Biochemical modes of action of Gmelilna asiatica inflammation. Indian Journal of Pharmacology, 1997;29(5) : 306-309
- Kumar, V.P. ., Chauhan, N.S., Padh, H.. and Rajini, M. B., Search for antibacterial and antifungal agents from selected Indian medicinal plants. Journal of Ethnopharmacology, 2006;107(2):182-8
- Merlin, N. J., Parthasarathy, V. and Santhoshkumar, T. R., Induction of apoptosis in human breast Cancer cell line MCF-7 by phytochemical from Gmelina asistica. African Journal of Biotechnology,2010; 9(28) : 4451-4456.
- Merlin, N. J., Parthasarathy, V., Manavalan, R., Devi, P. and Meera, R.,Phyto-Physico chemical evaluation, Anti-Inflammatory and Anti microbial activities of Aerial parts of Gmelina asiatica. Asian J.reasearch Chem,2009; 2(1) : 76-82.
- Parekh, J., Jadeja, D. and Chanda, S., Efficacy of aqueous and methanol extracts of some medicinal plant for potential antibacterial activity. Turkish Journal of Biology,2005; 29 : 203- 210.
- Rahman, M.M., Khurshid Alam, A.H.M., Sadik, G., Islam, M.R., Khondkar, P. Hossain, M.A. and Rashid, M.A.,Antimicrobial and cytotoxic activities of Achyranthes ferruginea. Fitoterapia,2007;78 (3): 260-262
- Rojas, J.J., Ochoa, V.J., Ocampo, S.A. and Munoz, J.F., Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine. African Journal of Traditional Complementary and Alternative Medicine,2006; 6:2.
- Satyanarayana, T., Katyayani, B.M., Hema, L.E., Routhu, K.V and Durga Prasad, Y., Phytochemical studies on root of Gmelina asiatica Linn. Pharmacognosy Magazine,2007; 3(11): 156-158.
- Sudhakar, M., Rao, Ch.V., Rao, P.M. and Raju, D.B., Evaluation of antimicrobial activity of Cleome viscosa and Gmelina asiatica. Fitoterapia, 2006b; 77(1): 47-49
- Sudhakar, M., Rao, Ch.V., Rao, P.M., Rajv, D.B., Evaluation of antimicrobial activity of cleome viscose and Gmelina asiatica. Fitoterapia,2006; 77(1) : 47-49.
- 15.Tanaka, H., Sato, M. and Fujiwara, S.,Antibacterial activity of isoflavonoids isolated from Erythrina variegate against methicillin resistant Staphylococcus aureus. Lett Appl Microbial, 2002; 35 : 494-498.
- 16.Teimouri, M., Korori, S., Moraghebi, F. and Matinizadeh, M.,Comparison of antibacterial activity of Quercus persia and Quercus ilex. South African Journal of Botany, 2004; 69:448-449.

This work is licensed under a Creative Commons Attribution 4.0 International License.





