How to Cite | Publication History | PlumX Article Matrix
Efficacy of Fungal Enzyme in Biodiesel Production from Vegetable Oil
M. N. Abubacker1 and A. Ayesha2
1Department of Biotechnology, National College, Tiruchirappali - 620 001, India. 2Department of Civil Engineering, Kongu Engg College, Perundurai, Erode - 638 052, India.
ABSTRACT: Biodiesel fuel (BDF) produced by alcoholysis of vegetable oils or fats is viewed as a promising renewable fuel source. Diminishing petroleum reserves and increasing environmental regulations have made the search for renewable fuel. Biodiesel is non-toxic and biodegradable, produced from renewable sources and contributes a minimal amount of net green-house gases, such as CO2, SO2 and NO emissions to the atmosphere. The main objective of the present study is to produce biodiesel from vegetable oil and to use micro-emulsions with solvents ethanol and methanol following acid, alkali and fungal enzyme catalysis methods. The best suited method of biodiesel production was ethanolic and alkali mediated trans-esterification process rather than methanolic and acidic trans-esterification. The maximum yield of biodiesel was obtained from Rhizopus oryzae lipase enzyme, ethanolic and alkali mediated trans-esterification followed by Aspergillus niger, Polyporus squamosus and Agaricus campestris.
KEYWORDS: Biodiesel; trans-esterification; Fungal enzyme; Lipase
Download this article as:| Copy the following to cite this article: Abubacker M. N, Ayesha AM. N. Efficacy of Fungal Enzyme in Biodiesel Production from Vegetable Oil. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Abubacker M. N, Ayesha AM. N. Efficacy of Fungal Enzyme in Biodiesel Production from Vegetable Oil. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9721 |
Introduction
The consumption and demand for petroleum products are increasing every year due to increase in population, standard of living and urbanization. Due to gradual depletion of world petroleum reserves and the impact of environmental pollution, there is an urgent need for suitable alternative fuels for use in diesel engines (Kloptenstem, 1988; Harrington, 1986). Today’s diesel engines require a clean burning and stable fuel that performs well under a variety of operating conditions. Biodiesel is the only alternative fuel that can be used directly in any existing unmodified diesel engine. Because it has similar properties to diesel fuel, biodiesel can be blended in any ratio with diesel fuel (Masjuki, 1993).
There are many reasons that justify the development of biodiesel as biofuel. It provides a market for excess production of vegetable oils, it decreases the dependence on imported petroleum, it does not contribute to global warming due to its closed carbon cycle, the exhaust emissions of carbon monoxide, unburned hydrocarbons and particulate emissions from biodiesel are lower than regular diesel fuel, when blended with added to crude oil derived diesel fuel upto 20% (Ayhau, 2009).
In view of this, vegetable oil is a promising alternative biofuel, which can be converted into biodiesel and it is produced easily in rural areas, where there is an acute need for modern forms of energy (LePori et al., 1992; Rao and Gopalakrishnan, 1991). In recent years systematic efforts have been made by several research workers to use vegetable oils as fuel in engines (Masjuki and Sohif, 1991; Nag and Bhattacharrya, 1995; Takeda, 1982; Piyaporn et al., 1996). Various oils like algal oil, sunflower oil, palm oil and olive oil have been used in different countries as raw materials for biodiesel production owing to its availability (Dorado et al., 2003; Hossain et al., 2008; Hossain and Boyce, 2009a, b). Biodiesel is defined as a fuel comprised of monoalkyl esters of long chain fatty acids derived from vegetable oils or animal fats (Vicente et al., 2007).
The high viscosity and poor volatility are the major limitations of vegetable oils for their utilization as fuel in diesel engines. High viscosity of vegetable oils deteriorate the atomization, evaporation and air-fuel mixture formation characteristics leading to improper combustion and higher smoke emission. Moreover this high viscosity generates operational problems like difficulty in engine starting, unreliable ignition and deterioration in thermal efficiency. Converting to biodiesel is one of the options to reduce the viscosity of vegetable oils (Pangazhabadivu and Jeyachandran, 2005). For this purpose fungal enzymes are used for trans-esterification process (Jin et al., 2008).
The first objective of this study aims to compare the optimum conditions of biodiesel production from commercial oil used for lighting lamps through trans-esterification process using alkaline and acidic catalysts. The second objective is to compare the efficacy of fungal enzyme through alkaline and acidic based trans-esterification process.
Materials and Methods
Materials – Oil
Commercially available ‘Deepam oil’ used for lighting lamps was purchased from a local grocery shop. The oil is a mixture of oils obtained from the seeds of Azadirachta indica A. Juss (neem), Meliaceae; Madhuca longifolia L. (Mowra – fat) Sapotaceae; Ricinus communis L. (Castor oil), Euphorbiaceae and Sesamum indicum L. (Gingelly oil), Pedaliaceae in equal proportions by volume.
GLC – Chromatograph of Standard Fatty Acid Methyl Ester Mixture of Commercial ‘Deepam Oil’ (Vegetable Oil)
The fatty acid composition of the vegetable oil used for this study was investigated by GLC (Gas Liquid Chromatography) after conversion of the acids into the corresponding methyl esters as described by Mangold and Kammereck (1961) and Loury (1967). The converted sample was injected into the column filled with 10% diethyl glycol succinate (DEGS) on 100-200 (British – Std. Sieve) mesh. The injector temperature was 230° C and the detector temperature was 250° C. Nitrogen gas was used as the carrier gas at a flow rate of 11.3 ml/min. Standard methyl esters peaks were identified with Sigma standards.
Fungi
The lipase enzyme was obtained from four different fungi viz. Agaricus campestris, Aspergillus niger, Polyporus squamosus and Rhizopus oryzae. A. niger and R. oryzae cultures were obtained from the Microbiology Lab, National College, Tiruchirappalli and A. campestris and P. squamosus were collected from natural source in College campus.
Fungal Lipase Extraction
Fungal lipase extraction was carried out according to Folch et al. (1957) method. The fungal mycelia in liquid medium or tissue was centrifuged at 10,000 rpm for 10 min. and the supernatant was discarded. The pellet was taken in 5.0 ml of methanol : chloroform in 2 : 1 ratio and kept in shaker for 20 min. then centrifuged at 10,000 rpm for 10 min. The organic phase was washed in 1 ml of water and again centrifuged at 2000 rpm for 5 min. The upper aqueous phase was removed and the lower organic phase was rinsed twice with 5.0 ml of methanol and water in 1:1 ratio. Finally the extracted lipid with lipase was collected from the solvent phase and stored for further experimental work and part of the lipase was crystallized and used further (Fig. 1).
Trans-esterification Reaction
Trans-esterification reaction process also called alcoholysis, is the displacement of alcohol from an ester by another alcohol in a process similar to hydrolysis except that an alcohol is used instead of water (Murugesan et al., 2009). This has been widely used to reduce the viscosity of the triglycerides. The trans-esterification is represented as:
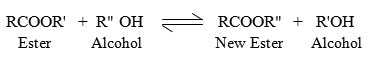
The trans-esterification reaction was performed by combining Deepam Oil with alcohol or methanol in the presence of a catalyst sodium hydroxide or hydrochloric acid and fungal enzyme. The esterification mixture consisted of 100 ml of Deepam oil, 20 ml of ethanol or methanol, 3 g of NaOH or 3 ml of HCl and 5 ml of fungal enzyme. The experiment was performed at 40° C and the reaction time was kept constant for 3 hours for all the experiments.
After trans-esterification reaction the biodiesel produced was separated from glycerol using separating funnel and finally washed with 5% water followed by magnesium sulfate anhydrous to remove the water. The biodiesel : glycerol ratio was recorded.
Biodiesel Analysis
Parameters like viscosity, acid number, carbon residue were analysed (Kalam and Masjuke, 2002) and compared with American Standard for Biodiesel Testing method (ASTMD 6751).
Results and Discussion
GLC – Chromatograph Studies of Vegetable Oil
The fatty acid composition of the vegetable oil used for biodiesel production revealed the presence of capric, caprylic, lauric, myristic, oleic, palmitic, nonanoic, steraric, undecanoic, arachidic and behenic acids identified as per the peak position and relative retention time of those standard methyl esters in GLC-Chromatograph.
Biodiesel Production
The results of biodiesel production by various combinations of esters, catalyst and fungal lipase enzyme mediated trans-esterification process are presented in Table-1.
Table 1: Biodiesel production at 40° C in 3 hours.
| Oil (ml) | Esters (ml) | Catalyst
(ml or g) |
Fungal lipase (ml) | Biodiesel (ml) | Glycerol (ml) |
| Vegetable oil (100) | Ethanol (20) | NaOH (3) | – | 40 | 60 |
| Vegetable oil (100) | Methanol (20) | NaOH (3) | – | 40 | 60 |
| Vegetable oil (100) | Ethanol (20) | HCl (3) | – | 35 | 65 |
| Vegetable oil (100) | Methanol (20) | HCl (3) | – | 35 | 65 |
| Vegetable oil (100) | Ethanol (20) | NaOH (3) | A. campestris (5) | 50 | 50 |
| Vegetable oil (100) | Methanol (20) | NaOH (3) | A. campestris (5) | 50 | 50 |
| Vegetable oil (100) | Ethanol (20) | NaOH (3) | A. niger (5) | 65 | 35 |
| Vegetable oil (100) | Methanol (20) | NaOH (3) | A. niger (5) | 60 | 40 |
| Vegetable oil (100) | Ethanol (20) | NaOH (3) | P. squamosus (5) | 55 | 45 |
| Vegetable oil (100) | Methanol (20) | NaOH (3) | P. squamosus (5) | 50 | 50 |
| Vegetable oil (100) | Ethanol (20) | NaOH (3) | R. oryzae (5) | 70 | 30 |
| Vegetable oil (100) | Methanol (20) | NaOH (3) | R. oryzae (5) | 65 | 35 |
Efficacy of fungal enzyme in Biodiesel Production
The maximum yield of 70 ml of biodiesel was obtained in 100 ml of oil with ethanol, NaOH and R. oryzae lipase mediated trans-esterification process. This is followed by A. niger (65 ml), P. squamosus (55 ml) and A. campestris (50 ml) lipase mediated trans-esterification process. The methanol-NaOH or methanol-NaOH-fungal lipase trans-esterification process in all the reactions produced lesser quantities of biodiesel (Table 1 and Fig. 1).
 |
Figure 1: Efficacy of Fungal Enzyme in Biodiesel Production from Vegetable Oil.
|
Komers et al. (2001) obtained biodiesel from rapeseed oil using methanol and KOH as catalyst. Mittelbach (1993) have produced biodiesel from vegetable oils. Enciner et al. (2002) produced biodiesel from vegetable oil of Cynara cardunculus. Mohamed and Ali (2002) produced biodiesel from palm oil. Zhang et al. (2003), Oliveira and Rosa (2006), Aranda et al. (2007), Kalam and Madjuki (2002), Demirbas (2007) and Hossain and Boyee (2009a) used sunflower oil and used cooking oil and produced biodiesel. All these results have shown variations in the output of biodiesel production from the present investigation. The variations are due to the triglyceride content of the oil, the type of fungal lipase and the type of transmethylation reaction during the production of biodiesel.
Pazonki et al. (2010), Kim et al. (2007) and Jin et al. (2008) used whole cell as well as Rhizopus oryzae and Candida rugosa lipase as biocatalysts in biodiesel-fuel production. The fungal lipase proved vital in catalylic activity of esterification process and generated high quality and quantity of biodiesel as reported by Kloptenstem (1988).
Biodiesel Analysis
Biodiesel analysis such as viscosity, acid number and carbon residue revealed 4.60, 0.20 and 0.021 respectively as against ASTMD 651, 1.9-6.0 mm2/sec at 4° C for viscosity, 0.5 mg KOH/g ASTMD 651 for acid number and < 0.3 EN 14214 for carbon residue.
Conclusion
The optimum conditions for biodiesel – fuel production from Deepam Oil showed that the oil may be employed as a substantial source of biodiesel as fuel in diesel engines. This research represented that the production of biodiesel from ethanol or methanol and catalyst NaOH or HCl has shown no significant differences in the biodiesel yield. However ethanol, NaOH and R. oryzae or A. niger lipase yielded significant quantity of biodiesel fuel which is considered as renewable energy.
Acknowledgement
We wish to thank DST-FIST, Government of India for instrumentation facilities provided to the Department of Botany, National College, Tiruchirappalli. The authors thank Sri. K. Ragunathan, Secretary and Dr. K. Anbarasu, Principal, National College, Tiruchirappalli for their encouragement. We also thank Dr. S. Kuppuswami, Principal, Prof. S. Krishnamoorthi, Head and Prof. N. Rajkumar, Department of Civil Engineering, Kongu Engineering College, Perundurai, Erode for their encouragement.
References
- Aranda, D. A. G., Santos, R. T. P., Tapanes, N. C. O., Ramos, A. L. D. and Antunes, O. C., Acid-catalyzed homogeneous esterification reaction for biodiesel production from palm fatty acids. Catalysis Letters, 122: 20-25 (2007).
- Ayhau, D., Progress and recent trends in biodiesel fuels. Energy Convers. Manage., 50: 14-34 (2009).
- Demirbas, A., Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Conv. Manag. 48: 937-941 (2007).
- Dorado, M. P., Ballesteros, E., Arnal, J. M., Gomez, J. and Gimenez, F. J. L., Testing waste olive oil methyl ester as a fuel in a diesel engine. Energy Fuels, 17: 1560-1565 (2003).
- Enciner, J. M., Gonzalez, J. F., Rodrignez, J. J. and Tajedor, A., Biodiesel fuel from vegetable oils: Trans-esterification of Cyanara cardunculus L. oils with ethanol. Energy Fuels, 16: 443-450 (2002).
- Folch, J., Lees, M. and Soloane-Stanley, G. H., A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biology and Chemistry, 226: 497-509 (1957).
- Harrington, K. J., Chemical and physical properties of vegetable oil esters and their effect on diesel fuel performance. Biomass, 9: 1-17 (1986).
- Hossain, A. B. M. S. and Boyce, A. N., Biodiesel production from waste sunflower cooking oil as environmental recycling process and renewable energy. Bulgarian Journal of Agricultural Science, 15: 313-318 (2009a).
- Hossain, A. B. M. S. and Boyce, A. N., Comparative study of biodiesel production from pure palm oil and waste palm oil. Arab Gulf J. Sci. Res., 27: 33-38 (2009b).
- Hossain, A. B. M. S., Salleh, A., Boyce, A. N., Prathim, P. and Naqiuddin, M., Biodiesel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 4: 250-254 (2008).
- Jin, G., Bierma, T. J., Hamaker, C. G., Rhykerd, R. and Loftus, L. A., Producing biodiesel using whole cell biocatalysts in separate hydrolysis and methanolysis reactions. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 43: 589-595 (2008).
- Kalam, M. A. and Masjuki, H. H., Biodiesel from palm oil: an analysis of its properties and potential. Biomass and Bioenergy, 23: 471-479 (2002).
- Kim, S. W., Lee, D. H., Lee, J. H. and Lim, J. S., Optimization for biodiesel production using a mixture of immobilized Rhizopus oryzae and Candida rugosa lipases. J. Biotechnol., 131: 123 (2007).
- Kloptenstem, W. E., Effect of molecular weights of fatty acid esters on cetane numbers as diesel fuels. J. Amer. Oil Chem. Soc., 65: 1029-1031 (1988).
- Komers, K., Stlonkal, R., Machek, J. and Skopal, F., Biodiesel from rapeseed oil, methanol and KOH, analysis of composition of actual mixture. Eur. J. Lipid Sci. Technol., 103: 363-371 (2001).
- LePori, W. A., Engler, C. R., Johnson, I. A. and Yarbrough, C. M., Animal fats as alternative diesel fuels, in liquid fuels from renewable resources. Proc. Alter Energy Conf. Amer. Soc. Agric. Eng., St. Joseph, 89-98 (1992).
- Loury, M., A general method for rapid conversion of fats to methyl esters. Rev. Franc. Corps. Gras. 12: 136-142 (1967).
- Mangold, H. K. and Kammereck, R., Separation, identification and quantitative analysis of fatty acids by thin-layer chromatography and gas liquid chromatography. Chem. and Ind., London, UK, pp. 1030-1040 (1961).
- Masjuki, H. and Sohif, M., Performance evaluation of palm oil diesel blends on small engine. J. Energy Heat Mass Transfer. 13: 125-133 (1991).
- Masjuki, H., Biofuel as diesel fuel alternative: An overview. J. Energy Heat Mass Transfer, 15: 293-304 (1993).
- Mittelbatch, M., Diesel fuel from vegetable oils. Gas chromatographic determination of free glycerol in transesterified vegetable oils. Chromatographia, 37: 623-626 (1993).
- Mohamad, I. A. W. and Ali, O. A., Evaluation of the trans-esterification of waste palm oil into biodiesel. Bioresource Technol., 85: 254-256 (2002).
- Murugesan, A., Umarani, C., Chinnusamy, T. R., Krishnan, M., Subramanian, R. and Nedunchezhain, N., Production and analysis of bio-diesel from non-edible oils: A review. Ren. Sust. Energy Rev., 13: 825-834 (2009).
- Nag, A. and Bhattacharrya, K. B., New utilization of vegetable oils. J. Amer. Oil Chem. Soc. 72: 1391 (1995).
- Oliveira, A. C. and Rosa, M. F., Enzymatic trans-esterification of sunflower oil in an aqueous oil biphasic system. J. Am. Oil Chem. Soc., 83: 21-25 (2006).
- Paugazhabadivu, M. and Jeyachandran, K., Investigations on the performance and exhaust emissions of a diesel engine using preheated waste frying oil as fuel. Renewable Energy. 30: 2189-2202 (2005).
- Pazouki, M. F., Zamani, A. H., Zamzamian, M., Fahar and Najafpour, G., Esterification of free fatty acids by Rhizopus oryzae as cell catalyzed from used cooking oil for biodiesel production. World Applied Sci. J., 8: 719-724 (2010).
- Piyaporn, K. J., Narumon and Knit, K., Survey of seed oils for use as diesel fuels. J. Amer. Oil Chem. Soc., 71: 471-477 (1996).
- Rao, P. S. and Gopalakrishnan, V. K., Vegetable oils and their methyl esters as fuels for diesel engines. Indian J. Technol., 29: 292-297 (1991).
- Takeda, Y., Developmental study on Jatropha curcas (Sabu dum) oil as a substitute for diesel engine oil in Thailand. J. Agri. Assoc. China., 20: 1-9 (1982).
- Vicente, G., Martinez, M. and Aracil, J., Optimization of integrated biodiesel production. Part-1. A study of the biodiesel purity and yield. Biores. Technol. 98: 1733-1742 (2007).
- Zhang, Y., Dube, M. A., Mclean, D. D. and Kates, M., Biodiesel production from waste cooking oil. 1. Process design and technological assessment. Biores. Technol., 89: 1-16 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





