How to Cite | Publication History | PlumX Article Matrix
New B-Carboline Alkaloid from Marine Sponge Hyrtios Reticulatus
Wafaa H. B. Hassan1,2
1Department of Pharmacognosy, King Saud University, Kingdom of Saudi Arabia.
2Department of Pharmacognosy, Zagazig University, Zagazig, Egypt.
Corresponding Author E-mail: wafhassan2004@yahoo.com
ABSTRACT: An Indonesian specimen of marine sponge Hyrtios reticulatus was found to contain a new b-carboline alkaloid [4-carboxy-6-hydroxy-b- carboline]. The structure was determined on the basis of spectral properties (ESI/MS/MS, UV and 1H NMR) and through comparison with the reported b-carboline alkaloids.
KEYWORDS: Hyrtios reticulatus, b-carboline alkaloid, antimicrobial; cytotoxic activity
Download this article as:| Copy the following to cite this article: Hassan W. H. B. New B-Carboline Alkaloid from Marine Sponge Hyrtios Reticulatus. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Hassan W. H. B. New B-Carboline Alkaloid from Marine Sponge Hyrtios Reticulatus. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9466 |
Introduction
Marine sponges of genus Hyrtios (class Demospongiae, order Dictyoceratida, family Thorectidae) (1-2) are rich source of structurally diverse metabolites including terpenoids, mainly sesterterpenes, and sesquiterpene quinones, macrolides, indole and β-carboline alkaloids (1-10). Many of these metabolites possess important biological activities as anticancer, cytotoxic, antiplasmodial, anti-inflammatory, antifungal, antineoplastic, antibacterial, antiproliferative activity and inhibition of HIV (1-14). Previous chemical investigation of H. reticulatus revealed the presence of novel derivative 1,6-dihydroxy-1,2,3,4-tetrahydro- β -carboline together with serotonin, 6-hydroxy-1-methyl-1,2,3,4-tetrahydro- β -carboline and 6-hydroxy-3,4- dihydro-1-oxo-β-carboline(5). In the present study the Indonesian sponge Hyrtios reticulatus was chemically investigated to isolate compound 1 from the biologically active butanol extract. The structure of the isolated compound was elucidated using UV, 1H NMR spectroscopy and mass spectrometry. The butanol extract showed mild growth inhibition activity against different cell lines including rat brain carcinoma (PC12) and human cervix cancer cells (HELA).
Experimental Section
Sponge Material
Marine sponge Hyrtios reticulatus, Thiele (reference no. TM21) was collected near the coast of Kapoposang island, Indonesia at a depth of 12 m, on August 1997 and immersed in ethanol immediately after collection. The specimen consisted of long orange brown-colored branches with side branches only very near the point of attachment. Several oscules up to 2 mm in diameter were present along the branches. Conules (3-4 mm apart) of 1-2 mm high were also present. The orange color was more enhanced at the apexes of the conules. The sponge was identified by Prof. Van Soest (Amsterdam) and a voucher specimen was kept in ethanol in the Zoological Museum Amsterdam under the registration number ZMA POR 14464.
Apparatus
Mass spectra recorded on Finningan MAT TSQ-7000 mass spectrometer, while 1H NMR spectrum was recorded on Bruker ARX 400 NMR spectrometer, UV spectrum was measured in methanol on a Perkin-Elmer UV/Vis lambda spectrophotometer. Solvents were distilled prior to use, and spectral grade solvents were used for spectroscopic measurements. TLC was performed on TLC plates pre-coated with silica gel F254 (Merck, Darmestadt, Germany). Semi-preparative HPLC was performed on HPLC system (Merck, Darmestadt, Germany) coupled with UV detector L7400 (UV detection at 280 nm), the separation column (8 x 250 mm) pre-packed with Eurosphere C18 (Knauer, Berlin, Germany). The compound was eluted with solvent system of MeOH/H2O containing 1% TFA for improved separation, at a flow rate of 5 ml/min.
Extraction and isolation
Specimens of H. reticulatus was freeze dried (50.9 g dry weight) and repeatedly extracted with MeOH (500 ml x 4) and the combined extracts were filtered and concentrated under reduced pressure to afford 12.4 g of crude extract. The residue was dissolved in water and methanol (9:1), adjusted to 300 mL before sequentially partitioning against hexane (extract, 1.8 g), Ethyl acetate (extract, 0.6 g) and butanol (extract, 1.1 g). The butanol extract was subjected to gel filtration chromatography using sephadex LH-20 column using MeOH as eluent to give 20 fractions. Fraction 10 was subjected to semi-preparative HPLC using water and methanol with 0.5% TFA. This led to the isolation of compound 1 with nearly 95 % degree of purity.
Compound 1
Was isolated as a yellow residue (2.5 mg). The UV λ max 216, 245, 284, 310 sh and 410 nm. The positive ESI-MS/MS [M + H]+ at m/z 229 fragments ion 201 [M+ – CHN], m/z 157 [M+ – C3H3O2], 175 [M+ – C3H3O+2H], m/z, 101 [M+ – 4H – C4H3O2N], and m/z 71 [C3H2O2+H or C4H4O+3H ]. The 1H NMR (DMSO-d6-400 MHz) data of 1 (Table 1).
Biology
Antimicrobial assay (15)
Agar plate diffusion assay was done using gram positive bacteria Bacillus subtilis 168, gram negative bacteria Echerichia coli (ATCC 25922) and two fungal strains: Cladosporium herbarum and Cladosporium cucumerinum. Sterile filter paper discs were impregnated with 20 µg of sample using methanol as the carrier solvent. The impregnated discs were then placed on agar plates previously incubated with the above strains. Control was done with methanol only. After incubation at 27ºC for 48 and 72 hrs, inhibition zone diameters were measured in mm.
Cytotoxicity assay (16)
Antiproliferative activity of butanol extract was examined against several cell lines rat brain carcinoma (PC12) and human cervix cancer cells (HELA) and was determined through MTT assay as mentioned earlier (16).
Result and Discussion
Chromatographic separation of the butanol fraction of Hyrtios reticulatus sponge afforded new β-carboline alkaloid (1).
Compound 1
The UV spectrum of compound 1 showed absorption bands at λ max 216, 245, 284, 310 sh and 410 nm suggested a conjugated indole moiety and this is closely related to the UV data of hyrtiosulawesine(5), The positive ESIMS analysis exhibited a protonated molecular ion peak at m/z 229 [M+ + H] for the molecular formula C12H8N2O3. While the positive ESI/MS/MS (Fig. 2) showed fragment ion at m/z 157 [M+ – C3H2O2-H] indicated the presence of a carboxylic acid functionality in the molecule adjacent to CH=. Other fragments at m/z 201 [M+ – CHN] indicated the presence of N=CH moiety. This was confirmed from the 1H NMR data (Table 1) which showed two downfield singlet signals at δH 8.80 (1H, s) and 8.90 (1H, s) for H-1 and H-3 respectively. The downfield shift of protons 1 and 3 indicated that the two protons are adjacent to hetero-atom =CH-N=CH-. The other mass fragment at m/z 101 for [M+– (C4H3O2N+4H)] confirmed the presence of COOH-C=CH-N=CH moiety in ring B. Furthermore the 1H NMR data of compound 1 showed two more downfield singlets at δH 12.54 and δH 11.83 for two exchangeable protons of the carboxylic OH and indolic NH-moieties, respectively. The presence of the phenolic OH moiety in ring A was evident from the 1H NMR (DMSO-d6) chemical shift at δH 9.30, The position of this OH at 6 was established by comparison of the 1H NMR signals at δH 7.14 (1 H, dd, 8.8, 1.9 Hz, H-7), 7.66 (1H, d, 1.9 Hz, H-5) and 7.51 (1H, d, 8.8 Hz, H-8) with those of Hyrtiosulawesine (2, Table 1)(5) which indicated that this compound possesses the same 6-hydroxy- β carboline moiety. And the substitution is not at position 7 as harmine derivative (3) or harmic acid (4) (4). The mass fragments at m/z 175 [M+– C3H3O-2H] and m/z 71 [C4H6O] confirmed the presence of free hydroxyl group at ring A. The above data confirmed with no doubt that compound 1 is 3-carboxy-6-hydroxy- β– carboline which is new compound.
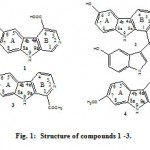 |
Figure 1: Structure of compounds 1 -3.
|
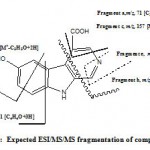 |
Figure 2: Expected ESI/MS/MS fragmentation of compound 1.
|
Antimicrobial assay
In this assay the butanol extract showed no activity against the used microorganisms.
Cytotoxicity Assay
In this assay the butanol extract showed mild growth inhibition activity against the used cell lines at concentration 50 μg/ml.
We could not carry out the biological activity for compound 1 due to the low yield.
Table 1: 1H NMR data of compound 1 -3
| No. | Compound 1 (DMSO, 400 MHz) | Compound 2 (MeOD)(5) | Compound 3 (DMSO-d6)(4) |
| 1 | 8.80 (1H, s) | – | 2.78 (3H, s, COCH3) |
| 3 | 8.90 (1H, s) | 8.41 (1H, d, 4.8 Hz) | 8.44 (1H, d, 5.0 Hz) |
| 4 | 12.54 (1H, s, OH) | 8.14 (1H, d, 4.8 Hz) | 8.24(1H, d, 5.0 Hz) |
| 5 | 7.66 (1H, d, 1.9 Hz)* | 7.56 (1H, d, 2.4 Hz,)* | 8.12 (1H, d, 8.2 Hz) |
| 6 | 9.30 (1H, s, OH) | – | 6.92 (1H, d, 8.2 Hz) |
| 7 | 7.14 (1 H, dd, 8.8, 1.9 Hz)* | 7.13 (1 H, dd, 8.5, 2.4 Hz)* | 3.83 (3H, s) |
| 8 | 7.51 (1H, d, 8.8 Hz)* | 7.53 (1H, d, 8.5 Hz)* | 7.35 (1H, d, 2.1 Hz) |
| NH | 11.83 (1H, s) | – | 11.65 (1H, s) |
* Comparable signals of ring A protons for both compounds
Acknowlegment
The author is very grateful to Prof. Peter Proksch for making the NMR analysis and antimicrobial activity and for Prof. Dr. W. E. G. Müller, Institut für Physiologische Chemiie, Abteilung Angewandte Molekularbiologie, Johannes Gutenberg-Univeritat, Mainz, Germany, for the Cytotoxicity assay. I also thank W.M. Van Soest, Head Section Invertebrates, Zoological Museum, University of Amsterdam, Amsterdam, for identification of the sponge.
References
- Sauleau, P., Martin, M. T., Dau, M.E.T.H., Youssef, D.T.A., Kondracki, M.L.B., Hyrtiazepine, an azepine –indole-type alkaloid from the red Sea marine sponge Hyrtios erectus, J. Nat. Prod. 2006; 69, 1676-1679.
- Ashour, M.A, Elkhayat, E.S., Ebel, R., Ederada, R., Proksch, P., Indole alkaloid from the Red Sea sponge Hyrtios erectus, ARKIVOC, 2007; XV, 225-231
- Lee, H.S., Yoon, K.M., Han, Y.R., Lee, K. J., Chung, S.C., Kim, T.I., Lee, S.H., Shin, J., Oh, K.B, 5- hydroxyindole-type alkaloids, as Canadida albican isocitrate lyase inhibitor, from the tropical sponge Hyrtios sp., Bioorganic & Medicinal Chemistry Letters, 2009; 19, 1051-1053.
- Hashimoto ,Y., Kawanishi, K., New alkaloids from Banisterpsis caapi, Phytochemistry, 1976; 15, 1559-1560.
- Salmoun, M., Devijver, C., Daloze, D., Braekman, J., Van Soest, R., 5- hydroxytryptamine-derived alkaloids from two marine sponges of genus Hyrtios, J. Nat. Prod., 2002; 65, 1173-1176.
- Salmoun, M., Devijver, C., Daloze, D., Braekman, J. C., Gomez, R., De Kluijver, M.,Van Soest, R.W.M., New sesquiterpene quinines from sponges of the Genus Hytrious, J. Nat. Prod., 2000; 63, 452-546.
- Tsuckiya, N., Sato, A., Hara, T., Sato, N., Sasagawa, K., Kobayashi, T., Cytotoxic scalarane sesterterpenes from a sponge, Hyrtios erectus, J. Nat. Prod., 1998; 61, 468-473.
- Ryu, G., Matsunaga, S., Fusetani, N., Three new cytotoxic sestertepenes from the marine sponge Hyrtios cf. erectus, J. Nat. Prod., 1996; 59, 515-717.
- Miyaoko, H., Nishijima, S., Mitome, H., Yamada, Y., Three New scalarane sesterterpene from the Okinawan sponge Hyrtios erectus, J. Nat. Prod., 2000; 63,1369-1372.
- Youssef, D. T. A., Yamaki, R.K., Kelly, M, Scheuer P.J., Salmahyrtisol, A., A novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erectus, J. Nat. Prod., 2002; 65, 2-6.
- Youssef, D., Hyrtioerectines A-C, cytotoxic alkaloids from the red Sea sponge Hyrtious erectus, J. Nat. Prod., 2005; 68, 1416-1419.
- Du , L., Shen, L., Yu, Z., Chen, J., Guo, Y., Tang, Y., Shen, X., Jiang, H.; Hyrtiosal, from the marine sponge Hyrtios erectus, inhibits HIV-1 integrase binding to viral DNA by a new inhibitor binding site, ChemMedChem 2008; 3, 173 – 180.
- Pettit, G.R., Tan, R, Melody N., Cichacz Z.A., Herald D.L,. Hoard M.S., Pettit R.K., Chapuis J.C., antineoplastic agents 397; Isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (the republic of Maldivie), Bioorg Med Chem Lett. 1998; 8(16), 2093-2098.
- Pettit, R.K., McAllister, S.C., Pettit, G.R., Herald, C.L, Johnson, J.M., Cichacz, Z.A.,a broad-spectrum antifungal from the marine sponge Hyrtios erectus, Int J Antimicrobial Agents. 1997; 9(3), 147-152.
- Hassan, W. ; Ederada, R., Ebel, R., Berge, A., Wray, V., Proksch, P., New alkaloids from Miditerranean sponge Hamigera hamigera; Mar. Drugs, 2004; 2, 88- 100.
- Kreuter, M.H., Robitzki, A., Chang, S., Steffen, R., Michaelis, M., Kljajic, Z., Bachmann, M., Schrooder, H.C., Muller, W.E.G., Proksch, P., Production of cytotoxic agent aeroplysinin by the sponge Veronga aerophoba in vitro culture, Comparative biochemical physiology, 1992; 101C, 183-187.

This work is licensed under a Creative Commons Attribution 4.0 International License.





