Manuscript accepted on : 11 February 2012
Published online on: --
Phytochemical Screening and Evaluation of In Vitro Antioxidant Activity of Mimosa catechu Bark
K. Ravishankar1* and Ch. Sandhya1
1Sri Sai Aditya Institute of Pharmaceutical Sciences and Research, Adb Road, Surampalem -533 437, India.
ABSTRACT: The world today is focusing more upon the herbal medication for curing various ailments.The trend of validating the plants with traditional medicinal usage is increasing.The present investigation deals with the phytochemical screening and evaluation of in vitro antioxidant properties of Mimosa catechu ethanolic bark extract. The In vitro antioxidant activity was evaluated using DPPH and Reducing power assay methods and the results are compared with Gallic acid as reference standard . Phytochemical screening of Ethanolic bark extract of Mimosa catechu showed the presence of various constituents like flavonoids, phytosterols, tannins and showed appreciable and concentration dependent in vitro antioxidant activity.
KEYWORDS: Mimosa catechu; Phytochemical screening; Antioxidant activity; DPPH radical scavenging
Download this article as:| Copy the following to cite this article: Ravishankar K, Sandhya C. Phytochemical Screening and Evaluation of In Vitro Antioxidant Activity of Mimosa catechu Bark. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Ravishankar K, Sandhya C. Phytochemical Screening and Evaluation of In Vitro Antioxidant Activity of Mimosa catechu Bark. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9725 |
Introduction
Active oxygen species and free radicals play an important role in the initiation and evolution of numerous diseases. The use of compounds with antioxidant activity is expected to be useful for the treatment of these diseases.Therefore, there has been a growing interest in finding novel antioxidants in order to meet the requirements of pharmaceutical industries1.Antioxidants are emerging as prophylactic and therapeutic agents for various diseases like cancer, diabetes, cardiovascular disorders, brain dysfunction, inflammation and other degenerative diseases owing to our sedentary way of life and stressful existence. Thus Antioxidants are the substances that delay or prevent the oxidation of cellular substrates.
Mimosa catechu Wild belonging to the family mimosacae is an important medicinal plant used in ayurveda for many diseases. It was reported that the heartwood of the plant is widely used in conjunctivitis, haemoptysis, catarrh, cough pruritis, leprosy, leucoderma, skin diseases, helmenthiasis, diarrhea, and dysentery, foul ulcers, wounds, fever, anaemia, diabetes, hemorrhages, chest diseases, asthma etc. A mixture of catechu and myrrh is usually prescribed as a galactogogue to women after confinement. It is also used as a hepatoprotective agent. The important chemical constituents [2] reported in the heartwood are catechin (2-12%), epicatechin [3], catechutannic acid (25-33%), catechin tetramer, dicatechin, gallocatechin, acacatechin (10-12%), catechuic acid, catechu red, kaempferol, taxifolin[4,5], isorhamnetin, (+) afzelechinn, L-arabinose, D-rhamnose, D-galactose, aldobiuronic acid, quercetin, phlebotannin (25-33%), quercetrin, quercitin. Very scanty work has been reported on Mimosa catechu bark ,hence we have selected the bark of this plant for the present study to explore its antioxidant properties.
The purpose of the present study was to carry out the phytochemical screening and to evaluate the in vitro antioxidant activity of bark of Ethanolic extract of Mimosa catechu .
Materials and Methods
Plant material
The plant material (bark) was collected from a village named Punyakshethram, which is about 20km away from Rajahmundry. The plant was identified and authenticated by the Botanist, Dr.T.U.Raghuram.
Preparation of the plant extract
The fresh bark of the plant was collected, dried under shade and powdered. Finally the dried powder was repeatedly extracted with absolute alcohol (maceration followed by hot percolation).
Chemicals and equipment
All the chemicals used are of analytical grade. DPPH (2,2-diphenyl-1-picryl hydrazyl) has been procured from RESEARCH-LAB FINE CHEM INDUSTRIES, MUMBAI. Gallic acid was a gifted sample from GLAXOSMITHKLINE. Potassium ferricyanide, Ferric chloride, Trichloro acetic acid, Sodium phosphate buffer has been procured from SIGMA CHEMICALS.
The different glassware used for the experimental purpose are of standard quality. UV-Visible double beam (ELICO SL 210), centrifuge machine and shimadzu electronic balance were used for the analysis.
Phytochemical Screening [6-7]
The Ethanolic extract of the bark was subjected to different qualitative tests for the identification of various possible constituents present in it. Thin layer chromatography of the alcoholic extract was also performed by using the solvent system of hexane and ethyl acetate in the ratio of 70:30. Concentrated sulphuric acid in methanol is used as the chromatographic spots detecting agent.
Antioxidant Activity
The antioxidant potential of the ethanolic bark extract of Mimosa catechu wild was investigated by employing the following methods.
Dpph Method [8]
The free radical scavenging activity was followed by preparing 0.002% DPPH solution in methanol. Gallic acid was taken as the reference standard. Different concentrations of the extract (25, 50 and 100 µg/ml) and standard drug (1 and 2.5 µg/ml) were prepared using methanol. 1ml of 0.002% DPPH solution is mixed with 1ml of all the concentrations of both extract and standard separately. These mixtures are kept in dark for about 30min and the optical density was measured at 517nm. 0.002% DPPH and methanol mixture is the blank. Finally the % inhibition of the DPPH activity is calculated using the formula.
DPPH Scavenged (%) = [ (Acontrol– Atest ) / Acontrol ] × 100
Where Acontrol is the absorbance of the control reaction and Atest is the absorbance in the presence of the sample of the extracts. The antioxidant activity of the Ethanolic bark extract was expressed as IC50 and compared with standard. The IC50 value was defined as the concentration (in μg/ml) of extracts that scavenges the DPPH radicals by 50%.
Reducing Power Assay [9]
Different concentrations of the extract (25, 50 and 100 µg/ml) and standard drug (1 and 2.5 µg/ml) were prepared using distilled water.1% potassium ferricyanide, 10% trichloro acetic acid, 0.1% ferric chloride and 0.2M phosphate buffer were prepared using distilled water. Gallic acid was taken as the reference standard. Then 1ml of each concentration of both extract and standard were taken separately and mixed with 1ml of 0.2M phosphate buffer (pH 6.6) and 1ml of potassium ferricyanide. Incubate all these samples at 500C for 20min. Then add 1ml of 10% trichloro acetic acid and centrifuge at 2000rpm for 10min. Now separate the upper layer (2.5ml) and then add (2.5ml) distilled water, 0.5ml of freshly prepared ferric chloride. Finally measure the absorbance at 700nm.
Results and Discussion
Phytochemical Screening
Qualitative screening of the ethanolic bark extract showed positive result for constituents which were displayed in the table1. In the TLC study, six major spots were observed in pink, purple and yellow colors indicating the presence of steroids, glycosides and flavonoids respectively. The results are displayed in the fig1.
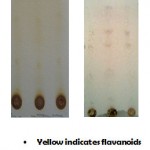 |
Figure 1 : TLC of Mimosa catechu.
|
Dpph Method
The reduction capability of DPPH radicals was determined by the decrease in its absorbance at 517 nm, which is induced by antioxidants. Table 2 shows the percentage of DPPH radical scavenged by Gallic acid and Ethanolic extract of bark at various concentrations (μg/ml). Figure 2,3 illustrates a decrease in the concentration of DPPH radical due to the scavenging ability of the soluble constituents in the Ethanolic extract of leaves of Mimosa catechu and the standard Gallic acid , as a reference compound, presented the highest activity at all concentrations. The IC50 values were found to be 8.6 µg/ml and 0.81 µg/ml for Ethanolic bark extract of Mimosa catechu and Gallic acid respectively.
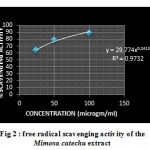 |
Figure 2 : free radical scavenging activity of the Mimosa catechu extract.
|
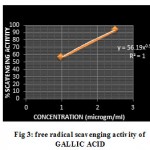 |
Figure 3: free radical scavenging activity of GALLIC ACID.
|
Table 2: Results of DPPH radical scavenging activity of ethanolic bark extract of Mimosa catechu
| TESTED
MATERIAL |
CONCENTRATION
(µg/ml) |
% INHIBITION OF
DPPH ACTIVITY |
IC50 (µg/ml) |
| Ethanolic bark extract of Mimosa catechu | 25
50 100 |
63.7±0.0007
79±0.0005 89±0.0007 |
8.6 |
| Gallic acid | 1
2.5 |
56.19±0.0125
93.4±0.0007 |
0.81 |
Values are the mean ± SEM., n=3
Reducing Power Assay
Reducing power assay is based on the principle that substances, which have reduction potential, react with potassium ferricyanide (Fe+3) to form potassium ferrocyanide (Fe+2),which then reacts with ferric chloride to form ferrous complex that has an absorption maximum at 700nm.The reducing capacity of a compound may serve as a significant indicator of it’s potential antioxidant activity[10]. Table 3 shows the reducing power of ethanolic bark extract of Mimosa catechu From figure 4,5 it was found that the absorbance of the extract increased with the increase in concentrations. Reducing power capabilities of extract was found to be closer to Gallic acid
Table 3: Results of Absorbance in Reducing power assay
| TESTED
MATERIAL
|
CONCENTRATION
(µg/ml) |
REDUCING POWER
(absorbance) |
| Ethanolic bark extract of Mimosa catechu | 25
50 100 |
0.443±0.019
0.6186±0.020 0.9136±0.026 |
| Gallic acid | 1
2.5 |
0.471±0.011 0.765±0.013 |
Values are the mean ± SEM., n=3
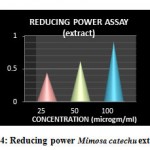 |
Figure 4: Reducing power Mimosa catechu extract.
|
 |
Figure 5: Reducing power of Gallic acid.
|
Conclusion
This research provides information which could trigger further research in the direction of partial or full isolation and characterization of the constituents of bark extract of Mimosa catechu in order to decipher the specific phytochemical constituent(s) responsible for the free radical scavenging activity of the plant. When this work is explored , Mimosa catechu could acquire important therapeutic application in phytotherapy. Experimental pharmacology confirming the Biological activities of Mimosa catechu extract in vivo system would be helpful to demonstrate the Rational use of this Plant .
Acknowledgement
The authors are thankful to the Vice Chairman Sri.N.Satish Reddy , for providing necessary facilities and constant encouragement through out the research work carried out in the Institution.
References
- Collins CA, Fry FH, Holme AL, Yiakouvaki A, Qenaei AA, Pourzand C and Jacob C,Org . Biomol. Chem. 2005 (3) :1541.
- L. Madhavi, D. K. Salunkhe, In D. L. Madhavi, S. S. Deshpande, D. K. Salunkhe, Editors,Food antioxidants, Dekker, New York,1996, 239.
- Gayathri Devi, Anitha john, R.Sreelekha devi, V.A.Prabhakaran, “Pharmacognostical studies on acacia catechu wild and identification of antioxidant principles”, International Journal of Pharmacy and Pharmaceutical Sciences, vol 3, suppl 2, 2011.
- Rao PR, Seshadri TR, L-Epicatechin from Acacia catechu, Journal Scientist Indian Research, 7B, 1948,59
- Bibhabasu Hazra, (Bose Institute), Kolkata, Rhitazit Sarkar, Santanu Biswas, Nripendranath Mandal, The Antioxidant, Iron Chelating and DNA Proyective properties of 70% Methanolic extract of ‘Katha’ (Heartwood extract of Acacia catechu) journal of complementary and integrative medicine vol.7/2010/issue 1.
- Wallis TE. Cutch. Text book of Pharmacognosy. 5th London: J. and A. Churchill ltd; 1967.
- Trease GE, Evans WC. Flavone and related flavomoid glycoside. Pharmacognosy. 4th London; Bailliere Tindall; 1972.
- Nayan Bhalidia.R, Pankaj Nariya.B. Acharya.R.N and Shukla V.J.2011. Evaluation of in vitro Antioxidant activity of flowers of Cassia fistula Linn. International journal of Pharm Tech Research, Vol. 3, No. 1, 589-599.
- Sonia Miladi., and Mohamed Damak.(2008).Invitro antioxidant activities of Aloe vera skin extracts. Journal de la Société Chimique de Tunisie,10, 101-109.
- Vishal DJ, Tekeshwar RV and Prajwal SR. Antioxidant potential of Bauhinia Purpurea Linn. Leaves. Int. J. Pharm. Research. 2009 (1) :51-55.

This work is licensed under a Creative Commons Attribution 4.0 International License.





