How to Cite | Publication History | PlumX Article Matrix
J. Ramesh Babu*, S. Vidyadhara and V. Anusha
Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Chandramoulipuram, Chowdavaram, Guntur - 522 019, India.
Corresponding Author E-mail: ramesh.janga11@gmail.com
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1064
ABSTRACT: A simple, specific, and accurate reverse phase high-performance liquid chromatographic method has been developed and validated for the simultaneous determination of Cefixime Trihydrate (CEF) and Ornidazole (ORD) in combined dosage form. Separation was performed on a C18 column [ODS UG 5 column, 250mm x 4.5mm], with methanol: water (50:50 v/v) isocratic elution with a flow rate of 1ml/min. Good sensitivity was observed with UV detection at 298nm. After method development, the influence of different parameters on the separation, the interference of other active compounds and excipients, repeatability and linearity were investigated. The retention times of CEF and ORD were 2.1 and 5.6min respectively and both the drugs showed good linearity in the concentration range of 2-10 µg/mL for CEF with a correlation coefficient (R) of 0.9996 and 5-25 µg/mL for ORD with correlation coefficient (R) of 0.9993 respectively. The proposed method was validated as per ICH guidelines and method showed good precision with percent relative standard deviation less than 2%. The percentage assay values of CEF and ORD were found to be 99.03 and 99.89% respectively and recovery values are within the limits of 98-102% indicating the proposed method was accurate and precise for the simultaneous estimation of CEF and ORD in bulk and pharmaceutical dosage forms.
KEYWORDS: Cefixime Trihydrate (CEF); Ornidazole (ORD); RP-HPLC; Validation.
| Copy the following to cite this article: Babu J. R, Vidyadhara S, Anusha V. Analytical Method Development and Validation for Simultaneous Estimation of Cefixime Trihydrate and Ornidazole in Tablet Dosage form by RP-HPLC. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Babu J. R, Vidyadhara S, Anusha V. Analytical Method Development and Validation for Simultaneous Estimation of Cefixime Trihydrate and Ornidazole in Tablet Dosage form by RP-HPLC. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10120 |
Introduction
Cefixime is an oral third generation cephalosporin antibiotic. Chemically, itis(6R,7R)-7-{[2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxyimino)acetyl]amino}-3ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid(Fig 1). It is used to treat gonorrhea, tonsillitis, and pharyngitis. ORD is an antimicrobial agent. Chemically it is 1-chloro-3-(2-methyl-5-nitro-1H-imidazol-1-yl) propan-2-ol (Fig 1).It is used in treatment of susceptible protozoal infections and anaerobic bacterial infection. Clinically study suggests that CEF is likely to be used in combination with other antimicrobial agents such as ORD, hence we developed RP-HPLC method for simultaneous estimation of both drugs in bulk and dosage forms.
Both the drugs are marketed as combined dose tablet formulation in the ratio of 200:500 mg CEF: ORD. Literature survey reveals that CEF can be estimated by spectrophotometrically, HPLC and by HPTLC individually or with other drugs in bulk drugs and in human plasma, while ORD can be estimated by spectrophotometrically, HPLC in combination with other drugs. However, there are only few analytical methods reported for the estimation of CEF and ORD in a combined dosage formulation. The present study is to develop a precise, accurate, simple, economical and specific RP-HPLC method to determine CEF and ORD in pharmaceutical dosage forms.
Materials and Methods
Equipment
Agilent 1120 compact LC chromatographic system, equipped with variable wavelength programmable UV detector and Rheodyne injector with 20 µL fixed loop was used for the chromatographic separation. The chromatogram was recorded and peaks were quantified by means of Ezchrome software. Chromatographic separation was carried out on a C18 column [Agilent ODS UG 5 column, 250mm x 4.5mm]. Elico double beam SL 218 UV-Visible spectrophotometer for spectroscopic determinations and Axis AGN 204-PO electronic balance was used for weighing purpose.
Chemicals and Reagents
Cefixime (CEF) and Ornidazole (ORD) were procured as gift samples from M/S Life Line Pharmaceuticals Ltd, Vijayawada. The tablet dosage form “MAHACEFTM” having combination of CEF (200mg) and OZD (500mg) were procured commercially from the local market. HPLC grade methanol and water procured from Merck, India.
Chromatographic Conditions
Mobile phase consisting of methanol: water (50:50 v/v) was used in isocratic mode. The mobile phase was initially filtered through 0.45μm millipore membrane filter and sonicated for 15 min before use. The flow rate was 1 mL/min and the injection volume was 20μL. UV detection was performed at 298nm and the separation was achieved at ambient temperature.
Experimental
Mobile phase composition:
Mobile phase consisted of Methanol and Water at 50:50 ratio was used for the present experimentation.
Preparation of standard stock solution
Accurately weighed quantities (10mg each of) CEF and OZD were dissolved separately in sufficient quantity of methanol in a 10ml volumetric flasks A and B. The volume was adjusted up to the mark with methanol to obtain a stock solution of mg/mL each of CEF and ORD. From the above standard stock solutions 1ml from volumetric flask A and 2.5 ml from volumetric flask B was transferred to a 100 ml volumetric flask and made up to the volume with same mobile phase to get 10µg/mL and 25µg/mL of CEF and ORD respectively.
Preparation of sample solution
The tablets were initially powdered and an amount equivalent to 4 mg of CEF and 10 mg of ORD was accurately weighed into a 10 mL volumetric flask, mixed with 5 ml of methanol and sonicated for 5 min with occasional shaking and made up to the volume with methanol. The solution was then filtered through 0.45µm millipore membrane filter and the 1000µl of filtrate was diluted with mobile phase to get a solution containing 6µg/mL and 15µg/mL of CEF and ORD respectively. The amount present in each tablet was calculated by comparing the areas of standard with the test samples.
Method Validation
The method was validated according to ICH Q2 B guidelines for validation of analytical procedures in order to determine system suitability, linearity, sensitivity, precision, accuracy and robustness for the analytes.
System suitability
System suitability was carried out by injecting 100% concentration (10µg/mL of CEF and 25µg/mL of ORD) into the HPLC system. This was repeated for six times under similar condition. The tailing factor (T) and no. of theoretical plates (N) obtained were given in
Table 1.
Linearity
Linearity of the method was determined by means of calibration curve using different concentration of the drugs. Linearity was evaluated by visual inspection of a calibration curve. The linearity of the method was determined in concentration range of 2-10µg/mL for CEF and 5-25µg/mL for ORD. Each solution was injected in triplicate. The proposed method was evaluated by its correlation coefficient and intercept value calculated in the statistical study. The calibration curves for CEF and ORD were shown in Figure 3a and 3b and their corresponding linearity parameters were given in Table 2.
Precision
Precision was studied by measuring intra-day (repeatability which was carried out by analyzing the drug solutions within same day) and inter-day (by injecting of samples over two consecutive days) variation of the method. Study was carried out by injecting six replicates of 100% concentration (10µg/mL of CEF and 25µg/mL of ORD) and the % RSD of the peak areas were given in Table 3.
Accuracy, as recovery
To confirm the accuracy of the proposed method, recovery experiments were performed by standard addition technique. In this method a known quantity of pure drug was added at three different levels i.e. 80 %, 100% and 120% to pre-analyzed sample solutions and calculated the recovery of CEF and ORD for each concentration. The results were given in Table 4.
Selectivity/Specificity
Selectivity is the ability of the method to produce a response for the analyte in the presence of other interferences, in order to prove that the method chosen was specific and selective. The parameters like retention time (Rt), resolution (RS), tailing factor were calculated and given in Table 1.
| Parameters | Cefixime | Ornidazole | Limit |
| Retention time (min) | 2.1 | 5.6 | |
| Theoretical plates (N)
2000
|
4073
|
13200
|
N >
|
| Tailing factor (T) | 1.2
2 |
1.4
|
T of <
|
| Resolution (Rs) | 3.5
2 |
|
Rs
of >
|
LOD and LOQ
The LOD and LOQ values were determined by the formulae LOD = 3.3 σ/S and LOQ = 10 σ/S (Where, σ is the standard deviation of the responses and S is mean of the slopes of the calibration curves) and were given in Table 2.
Table 2: Results for Linearity (n=3).
| Parameters Cefixime | Ornidazole | |||
| Slope 657368 | 631991 | |||
| Intercept 30243 | 77309 | |||
| Correlation co-efficient 0.9996 | 0.9990 | |||
| Regression equation | y = 657368x – 30243 | y = 631991x -77309 | ||
| Linearity range 2-10µg/ml | 5-25µg/ml | |||
| LOD 0.16 µg/ml | 0.33 µg/ml | |||
| LOQ 0.49 µg/ml | 1.01 µg/ml |
Robustness
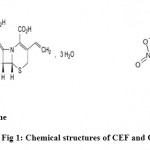 |
Figure 1: Chemical structures of CEF and ORD.
|
The robustness of the method was investigated under a variety of conditions including changes in the flow rate and detection wavelength and the % RSD was given in Table 5.
Result and Discussion
The scope of the present work was to optimize the chromatographic conditions. To develop RP-HPLC method for the estimation of CEF and ORD in selected multicomponent dosage forms. A binary mixture of methanol: water (50:50 v/v) as mobile phase was proved to be most suitable of all the combinations since the chromatographic peaks obtained were better defined and resolved and free from tailing. The developed method was also validated. The retention times obtained for CEF and ORD were 2.1 and 5.6 min respectively. An optimized chromatogram showing the separation of CEF and ORD at different Rt’s were shown in Figure 2
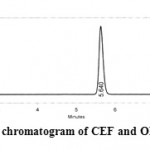 |
Figure 2: Optimized chromatogram of CEF and ORD by RP-HPLC.
|
System suitability was carried out by injecting 100% concentration of CEF and ORD six times into the HPLC system. The tailing factor was less than 2 and theoretical plate number was more than 2000 for both the drugs which was within the limits. The parameters were given in Table 1.
The linearity and range was found be in the range of 2-10µg/mL for CEF and 5-25µg/mL for ORD. The correlation coefficient of CEF and ORD were found to be 0.9996 and 0.9990 respectively and thus indicated the good linearity in the specified concentration range. The results were given in Table 2.
Precision was reported as % RSD and given in Table 3. The minimum variation in the %RSD values obtained indicated the present method is precise.
Accuracy of the proposed method was assessed by standard addition method at 80%, 100% and 120% levels of recovery to the preanalysed sample in triplicate. Recovery values obtained were given in Table 4 indicated that the proposed method was highly accurate. The recovery of the added standard to the drug product sample was calculated and was found to be 99.85 to 100.35% for CEF and 99.23 to 101.7% for ORD. The % RSD was less than 2 for both the drugs which indicated a good accuracy of the method to that of the label claim.
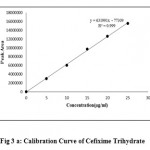 |
Fig 3 a: Calibration Curve of Cefixime Trihydrate.
|
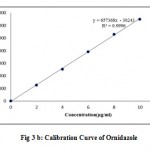 |
Figure 3 b: Calibration Curve of Ornidazole.
|
The method specificity was assessed by studying the chromatograms obtained from the sample solution. The method was found to be specific as none of the excipients interfered with the analytes of interest which was shown in Figure 4. Hence, the method was found to be suitable for analyzing the commercial antiretroviral formulations.
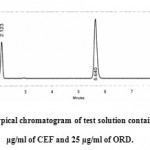 |
Figure 4: A typical chromatogram of test solution containing 10 μg/ml of CEF and 25 μg/ml of ORD.
|
LOD and LOQ were calculated from the average slope and standard deviation of y-intercepts of the calibration curve. LOD was found to be 0.16 µg/mL and 0.33 µg/mL respectively for CEF and ORD, LOQ was found to be 0.49 µg/mL and 1.01µg/mL respectively for CEF and ORD indicated high sensitivity of the method. The results were given in Table 2.
Robustness was carried out by change in the flow rate (±0.1mL/min) and variation in wavelength (± 2 nm). Solution of 100% concentration is prepared and injected in triplicate for every condition and % R.S.D calculated was found to be less than 2 for each condition. The results were given in the Table 5.
Assay of marketed formulation
20µl of sample solution was injected into chromatographic system and the peak responses were measured. The solution was injected three times into the column. The amount present in each tablet was calculated by comparing the areas of standards with that of the test samples and found to be 99.03 % w/w and 99.89 %w/w for CEF and ORD respectively. A typical chromatogram of test solution containing 6 μg/ml of CEF and 15 μg/ml of ORD was shown in Figure 4. The results were given in Table 6.
Conclusion
The developed and validated RP-HPLC method was found to be economical due to the use of higher percentage of water as a solvent in mobile phase. The low solvent consumption (1mL/min), along with short analytical run time of less than 10.0 minutes lead to an environmental friendly chromatographic procedure that allows the analysis of a large number of samples in a short period of time. This method has been found to be better than previously reported methods, due to its wider range of linearity, use of readily available mobile phase, lack of extraction procedures. Hence above method can be used in quality control for routine analysis of finished products of CEF and ORD without any interferences.
Acknowledgement
The authors are thankful to the management of Chebrolu Hanumaiah Institute of Pharmaceutical Sciences, Guntur (Andhra Pradesh) for providing facilities to carry out
research work.
References
- The Merck Index, 14th edition, Pg.no: 315, 1184.
- Levi-Comp’s Drug Information Handbook International, 13th Edition by Charles F. Lacy, Lora L. Armstrong, Morton P. Goldman, Leonard L. Lance, Pg.no: 293-294,1177, 2005.
- Indian Pharmacopoeia, Vol. II, Pg.no: .1012-1014, 2010.
- Indian Pharmacopoeia, Vol. II, Pg.no: 1823-1825, 2010.
- The United States Pharmacopoeia National Formulary- Official Compendia of Standards, Vol-II, Pg.no: 1654-1657, 2007.
- D.V.Madhura, G.T.Vandana, and J.P.Pranav, Simultaneous Estimation of Cefixime Trihydrate and Erdosteine in Pharmaceutical Dosage Form by Using RP-HPLC, International Journal of ChemTech Research,2(1),2010.
- M.Sudha, P.Surender, G.Mubeen, G.Divakar and S.Ravinder, A Novel HPLC-PDA Method Development and Validation for the Simultaneous Estimation of Cefixime and Ambroxol HCL in Tablets, International Journal of Pharmaceutical Sciences,3(2), 2011.
- Sudhakar, J.Venkateswara Rao,GS.Devika and R.P Ramesh, A V alidated RP-HPLC Method for Simultaneous Estimation of Cefixime Trihydrate and Ornidazole in Tablet Dosage Form, International Journal of Chemical and Pharmaceutical Sciences,1(2),2010.
- N.A.Khalaf, A.K.Shakya, M.Shurabji and D.Goindi, Development and Validation of an RP-HPLC Method for Simultaneous Analysis of Ofloxacin and Ornidazole in Tablets, Jordan Journal of Pharmaceutical Sciences 3(2), 2010.
- Nanda, J.Gaikwad and Prakash A, Simultaneous Spectrophotometric Estimation of Ornidazole and Cefixime in tablet dosage form, Internatinal Journal of PharmaTech Research, 1(3), 2009.
- K.Kapil, S.V.Gandhi, D.B.Padmanbh and N.V.Gaikwad, A Simple and Sensitive RP-HPLC Method for Simultaneous Estimation of Cefixime and Ofloxacin in Combined Tablet Dosage Form, International Journal of Pharmacy and Pharmaceutical Sciences, 3(1), 2011.
- C Brittain, et al. The Pharmacokinetic and Bactericidal Characteristics of Oral Cefixime, Clin Pharmacol Ther 1985:35:590-4.
- E.Schwartz, F.Jeunet, Comparative Pharmacokinetic Studies of Ornidazole and Metronidazole in man. Chemotherapy 1976; 22: 19-29.
- Sarode, D.R.Chaple, A.P. Nikhade and M.Yeole, “Development and Validation of RP-HPLC Method for Simultaneous Estimation of Cefixime and Ornidazole in Tablet Dosage Form”, International Journal of Universal Pharmacy and Life Sciences, Vol.2 (4):258-263, 2012.

This work is licensed under a Creative Commons Attribution 4.0 International License.





