How to Cite | Publication History | PlumX Article Matrix
Najat Marraiki
Botany and Microbiology Department, College of Science (Girls Section), King Saud University, Kingdom of Saudi Arabia. Corresponding Author E- mail: najat@ksu.edu.sa
DOI : http://dx.doi.org/ http://dx.doi.org/10.13005/bbra/1033
ABSTRACT:
Over the past decade metallic nanoparticles (NPs) have been intensively studied due to their distinctive chemical and physical properties. With the continuous rise in antibiotic-resistant bacteria, it became necessary to search for and develop antibacterial agents capable of effectively destroying bacteria with limited side effects. The antibacterial properties of metal oxide nanoparticles such as TiO2 and ZnO open up the wide application of such agents in a huge range of industries including but not limited to food packaging, food equipment manufacturing, and food formulation. Whilst their toxicity to humans is not yet well established, the mechanisms of action by which these metal nanoparticles disrupt bacterial cells are important to understand.
KEYWORDS:
Metallic Nanoparticles;Antibacterial activity; Nano-Metals, Toxicity
| Copy the following to cite this article: Marraiki N. Antibacterial Nano-Metals. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Marraiki N. Antibacterial Nano-Metals. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9929 |
Introduction
Metallic nanoparticles (NPs) have drawn a lot of attention, due to their unique characteristics and their promising applications in optical, electrical, mechanical, chemical and medical fields. They also have the ability to behave as antiviral agents [1 – 3]. Knowledge of the toxicity of NPs to human health is not fully complete but NPs are considered toxic and are classified into four different categories: (i) Nanomaterials based on metallodendrimers. Metallodendrimers composed of nanosized metals and are conjugated with organic polymers are typically used in molecular electronics and catalysis [4, 5]. (ii) Carbon-based nanomaterials made of carbon nanotubes or fullerenes. Such accumulate in living cells causing cytotoxicity and pulmonary toxicity [6 – 9]. (iii) Metal based nanomaterials, including quantum dots and various metallic NPs such as Ag, Au, and Pt NPs and Metal oxides, such as Al2O3, CrO3, Fe3O4, SiO2, TiO2 and ZnO2. Such metal oxides have been found to reason adverse effects causing conditions such as oxidative stress, inflammation of endothelial cells, apoptosis and ecotoxicity [10 – 13]. (iv) Metal NPs composites that combine a few different NPs to form bulk-type materials such as Fe-Pt NPs. Such composites have the power to cause mutagenicity in cells like bacterial cells [14].
Properties of NPs
The chemical and physical properties of NPs differ greatly from those of their corresponding bulk. Their sizes can range within the nanoscale up to 100 nm.
Nanomaterials have been found to have many different shapes and structures. They can exist as spheres, plates, tubes, needles, sheets, etc. Their distinct sizes and shapes contribute to their toxicity, for instance, single-wall nanotubes are more toxic than multi-wall nanotubes [15, 16]. In order to characterise NPs, it is important to fully understand their physicochemical properties; (a) size distribution, (b) shape, (c) structure, (d) their nature to agglomerate/aggregate, (e) surface area, (f) surface charge, (g) surface chemistry and (h) porosity [17]. Many methods have been employed to investigate these properties, including the uses of scanning and transmission electron microscopes (SEM & TEM), X-ray diffraction, zeta potential, spectroscopic techniques (i.e. UV, IR, etc.), isothermal absorption and dynamic light scattering (DLS) [17].
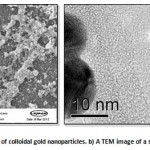 |
Figure 1: a An SEM image of colloidal gold nanoparticles. b) A TEM image of a single gold nanoparticle.
|
Some nanoparticles rely on illumination or on the application of a magnetic field to generate heat [18]. For example, light-absorbing gold nanoparticles can be attached to bacteria, which allows the use of lasers to target the bacteria to be killed [19].
If a system lacks certain conditions, then the NPs may aggregate and form their corresponding bulk. They tend to aggregate by fusion and deposition. NPs in gases usually agglomerate faster than they do when in liquid. They form primary agglomerates as primary free NPs stick to each other via interparticle interactions such as weak van der Waals, electrostatic and sintered bonds [20]. Particle-particle interactions that govern the nanoscale are van der Waals, stronger polar and covalent or electrostatic interactions. Viscosity and polarisability of the aqueous environment also influence these interactions in order to form agglomerates. Attractive and repulsive forces determine the states of individual and collective NPs.
Many of today’s NPs are modified chemically or are engineered by surface modification that will hence avoid agglomeration. In the presence of certain chemical agents, surfaces of NPs are allowed to become greatly modified so that they are indirectly stabilised against coagulation [17, 21].
Demonstration of Potential Hazard to Bacteria
Currently addressed concerns are related to human cells or health. However, impacts of NPs that exist on other living organisms are important for discussion, such as those on prokaryotes like bacteria and many other unicellular microorganisms. Unicellular microorganisms can be used as models for testing NP-toxicity analysis, for they are more sensitive than human fibroblast [22, 23].
Studies have been performed on isolated cultures and not on microbial communities that can exhibit more complex and antagonistic interactions. Some substances have been established already as being antimicrobial agents, such as TiO2 nanoparticles that work by inhibiting fouling processes by E. coli when the system is placed under UV illumination [24].
NPS enter microorganisms in their food, and become parts of their food chain. This, eventually, leads to the disrupting of the ecological balance, causing cellular toxicities at certain levels. Entry of NPs into these microorganisms takes place via various means, of which include physical rupturing of cell membrane, wall or endocytosis. It has been reported that metallic NPs have the ability of either passing through to the cell cytoplasm or remain attached to the cell surface membrane [25, 26].
Bacterial cells can also undergo lysis if their cell walls are damaged. Research has demonstrated that metal NPs are capable of damaging bacterial cell walls as they release ions. This causes increased membrane permeability, loss of the proton motive force and efflux of intracellular components [27]. Nanoparticles first attach and anchor the surface of bacterial cell walls. Large numbers of electrostatic forces and molecular interactions are involved at this first stage of interaction [28, 29]. Damages caused at this stage include structural and morphological changes. Any damages can also result in cell death [29, 30].
Nanoparticles tend to associate with enzymes, membrane porins, chaperones or periplasmic peptide-binding proteins of bacterial cell wall. And damage can lead to alterations and eventually affect bacterial enzymatic activities. This may result in morphological changes such as filamentation and DNA coagulation [28].
Different toxicities of NPs are exhibited, which are dependent on two main aspects: (i) the nature of NPs, including their size, morphology and chemical properties. (ii) NPs’ different interactions with different microbial species. The activities are also dependent on the type of bacterial organisms and can be highly related to their cell wall structures and their outer membrane arrangements. This is because significant differences exist between gram-positive bacteria and gram-negative bacteria. Outer membranes and unique periplasmic spaces are exhibited by gram-negative bacteria but not by gram-positive bacteria [28, 31].
Disaggregation of the exopolysaccharide matrix, cell separation, elongation and formation of small clusters are produced at NPs first interaction with the bacterial cell walls.
The disruption of cell membrane becomes more predominant at the second phase of interaction between NPs and bacterial cell walls. The cell wall perforates and thickens [32].
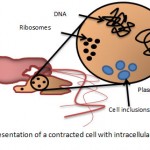 |
Figure 2: a schematic representation of a contracted cell with intracellular material being released.
|
Toxicity assays of NPs using bacteria as models
Microorganisms exist in almost every environment available on earth. Their flexibility and adaptability allows them to survive under extreme conditions such as anaerobic, high heat conditions. As a whole, they produce the majority of the biomass in aquatic systems. Plants are the primary biomass in terrestrial systems, but their survival, however, is dependent on the activity of microorganisms for the breakdown of dead matter and hence the recycling of needed nutrients. Microorganisms play a major part in the cycling of carbon, phosphorus, nitrogen and other useful minerals. Microorganisms are also essential components of soil health and have the features that will allow them to serve as potential mediators of transformations. These will affect nanoparticle mobility and toxicity in the external and internal environment. Evaluation of microbial toxicity allows extrapolation of the observed effects of chemicals on microorganisms to other higher level organisms in the food chain/web.
For convenience, the Quantitative Structure Activity Relationships (QSARs) method is used to calculate any impact on other organisms based on chemical structure. This method joins mathematical relationships between the structure of chemicals and their likelihood to increase the toxicity of compounds. A numerical value is assigned to each chemical and the sum of its compound gives the overall toxicity. Similar calculations can also be used to extrapolate the toxicity of a chemical to an organism based on its toxicity to an unrelated organism [33].
To investigate the detrimental effects of NPs on different organisms along with their impacts and mode of action, specific microorganisms can be used in toxicity assays. It has been reported that there are two major effects of NPs on bacteria. NPs are able to encourage oxidative toxicity in addition to damaging the cell membranes of many if not all cells. Currently, however, modes of action of these NPs in bacterial cells are not yet fully understood.
Assessing toxicity of NPs using bacteria as the target cell is very helpful in allowing the understanding of many of the concepts involved. Bacterial assays are faster, cheaper, more sensitive, and are easily handled compared to, for example, mammalian cells. Different NPs all possess different toxicity levels and they are all dependant on many of the morphological features associated with the difference in involved atoms. Therefore, they will all have different effects on living cells. Likewise, they will all have different effects on different cells [34]. It is just that today’s knowledge on the toxicity of NPs is insufficient to allow the identification of any systematic rules that govern the toxicological properties of all the products of nanotechnology [35].
The biocidal effectiveness of metal nanoparticles has also been suggested to be a result of both their size and their high surface-to0-volume ratio. These characteristics allow the NPs to interact with bacterial membranes [36].
Mechanism of Action
The mechanisms by which nanoparticles work by are not yet fully understood, however, there are three proposed mechanisms that are widely accepted today. (1) Cells uptake metal ions causing depletion of intracellular ATP production and disruption of DNA replication [37]. (2) Reactive oxygen species generated from metal NPs and metal ions followed by oxidative damage to cellular structures [31] as shown in figure 3. (3) The accumulation and dissolution of metal NPs in the bacterial membranes leading to the permeability changes and the dissipation of the proton motive force [38, 39].
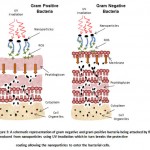 |
Figure 3: A schematic representation of gram negative and gram positive bacteria being attacked by ROS produced from nanoparticles using UV irradiation which in turn breaks the protective coating allowing the nanoparticles to enter the bacterial cells.
|
Silver nanoparticles: Silver nanoparticles cause envelope protein precursors to accumulate and the proton motive force to dissipate. Silver nanoparticles work by destabilising the outer membrane, cause the potential of the plasma membrane to fall rapidly and cause the levels of intracellular ATP to decrease [37].Silver nanoparticles are revealed to accumulate in the cell membranes and some penetrated into the cells [40].
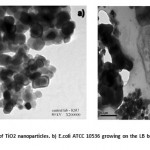 |
Figure 4: a A TEM image of TiO2 nanoparticles. b) E.coli ATCC 10536 growing on the LB broth after 4h with TiO2 nano-particles.
|
Gold nanoparticles: Gold nanoparticles are biocompatible and are exploited in organisms [41, 42]. Nanomaterials based on gold, such as gold nanorods, gold nanoshperes and gold nanocages, have the capability of destroying cancer cells and bacteria via chemotherapy [43, 44]. Antibacterial activities of gold nanoparticles can be enhanced by adding antibiotics [45].
Magnesium oxide nanoparticles are usually prepared through an aerogel procedure producing what is known to be AP-MgO with square and polyhedral shaped nanoparticles of diameters of around 4nm [46]. One of their key properties is their ability to adsorb and retain for a long time huge amounts of elemental chlorine and bromine [47]. AP-MgO also exhibits biocidal activity against some gram-negative bacteria and spores. Their high surface area and enhanced surface reactivity allows the magnesium oxide nanocrystals to adsorb and carry a significant amount of active halogens. Many particles are allowed to cover the bacteria cells to a high extent because of their extremely small sizes. This allows them to bring the halogens to an active form [48].
Zinc oxide nanoparticles have been found to be very toxic. They are stable under harsh processing conditions and have potent antimicrobial properties [49]. Some studies have shown that zinc oxide nanoparticles have selective toxicity to bacteria and minimal effect on human cells [50 – 53]. They have been reported to inhibit the growth of certain bacteria by disintegrating the cell membrane and increasing the membrane permeability [54]. It has also been reported that the hydrogen peroxide generated from the surface of the zinc oxide is an effective means for the growth inhibition of bacteria [55]. In other studies, zinc oxide nanoparticles have been found to release Zn2+ ions that damage the cell membrane and interact with intracellular contents [50].
TiO2 particles are catalysts that can catalyse the killing of bacteria using UV light at wavelengths of less than 385 nm [56 – 58]. Photo-excited TiO2 particles can generate active free hydroxyl radicals, leading to the antimicrobial effect of TiO2[59 – 61]. Nanostructured TiO2 on UV radiation can be used as an effective means for reducing infection time by eliminating pathogens on food surfaces [56].
Copper oxide nanoparticles are not fully investigated to give sufficient information on their antimicrobial effects, however, copper oxide is cheaper than silver and finds a wide application [62]. They have extremely large surface area and unusual crystal morphologies as a result of how they are prepared and so find potential application as antimicrobials [49]. However, high concentrations of copper oxide nanoparticles are needed to achieve bactericidal effects [63].
Aluminium nanoparticles carry a positive charge on its surface at near-neutral pH. Electrostatic attraction is produced between the positive alumina nanoparticles and the negative E. coli cells, causing the particles to adhere to the cells on the bacterial surfaces [64]. Adhesion increases with nanoparticle concentration. With this, there was a negative correlation between bacterial growths against nanoparticle concentration [65]. Antimicrobial activity of the alumina oxide is attributed to the production of reactive oxygen species (ROS), that causes the disruption of cell walls and consequently cell death [66].
References
- Marchesan, S.; Da Ros, T.; Spalluto, G.; Balzarini, J.; Prato, M. Anti-HIV properties of cationic fullerene derivatives. Bioorganic Medicinal Chemistry Letters. 2005, 15, 3615–3618.
- Piotrovsky, L.; Dumpis, M.; Poznyakova, L.; Kiselev, O.; Kozeletskaya, K.; Eropkin, M.; Monaenkov, A. Study of the biological activity of the adducts of fullerenes with poly(N-vinylpyrrolidone). Molecular Materials.2000, 13, 41–50.
- Schinazi, R.; Sijbesma, R.; Srdanov, G.; Hill, C.; Wudl, F. Synthesis and virucidal activity of a water-soluble, configurationally stable, derivatized C60 fullerene. Antimicrobial Agents and Chemotherapy.1993, 37, 1707–1710.
- ]AstrucD, Blais JC, Daniel MC, Gatard S, Nlate S and Ruiz J.Metallodendrimers and dendronized gold colloids as nanocatalysts, nanosensors and nanomaterials for molecular electronics. C R Chim. 2006, 6, 1117-1127
- Caminade AM and Majoral JP. Nanomaterials based on phosphorus dendrimers. AccChem Res. 2004, 37, 341-348
- Lam CW, James JT, McCluskey R and Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation.Toxicol Sci. 2004, 77, 126-134
- Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, Catsicas S, Schwaller B and Forro L. Cellular toxicity of carton-based nanomaterials. Nano Lett. 2006, 6, 1121-1125
- Porter AE, Muller K, Skepper J, Midgley P and Welland M. Uptake of C60 by human monocyte macrophages, its localization and implications for toxicity: studied by high resolution electron microscopy and electron tomography. ActaBiomater. 2006, 2, 409-419
- Wei W, Sethuraman A, Jin C. Monteiro-Riviere NA and Narayan RJ. Biological properties of carbon nanotubes.J NanosciNanotechnol. 2007, 7, 1284-1297
- Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, Schins R, Stone V, Kreyling W, Lademann J, Krutmann J, Warheit D and Oberdorster E. The potential risks of nanomaterials: a review carried out for ECETOC. Part FibreToxicol. 2006
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM and Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect. 2007, 115, 403-409
- Heinlaan M, Ivask A, Blinova I, Dubourguier HC and KahruA. Toxicity of nanosized and bulk ZnO, CuO and Ti0(2) to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalusplatyurus. Chemosphere. 2008, 71, 1308-1316
- Jeng HA and Swanson J. Toxicity of metal oxide nanoparticles in mammalian cells. J ErvironSci Health A Tox Hazard and Subst Environ Eng. 2006, 41, 2699-2711
- Subramanian V, Wolf EE and Kamat PV. Influence of metal/metal ion concentration on the photo catalytic activityof Ti02-Au composite nanoparticles. Langmui., 2003, 19, 469-474
- Jia G, Wang H, Van L, Wang X, Pei R, Yan T, Zhao Y and Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005, 39, 1378-1383
- Kang S, Pinault M, Pfefferle LD and Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007, 23, 8670-8673
- Oberdorster G. Oberdorster E and Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspec. 2005, 113, 823-839
- Hilger, I.; Hiergeist, R.; Hergt, R.; Winnefeld, K.; Schubert, H.; Kaiser, W. A. Thermal ablation of tumors using magnetic nanoparticles—An in vivo feasibility study. Investigative Radiology.2002, 37, 580–586.
- Zharov, V. P.; Mercer, K. E.; Galitovskaya, E. N.; Smeltzer, M. S. Photothermalnanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophysical Journal.2006, 90, 619–627.
- Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D and Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Past FibreToxicol. 2005a, 2, 8
- Oberdorstor G. Stone V and Donaklson K. Toxicology of nanoparticles: a historical perspective. Nanotoxicology. 2007, 1, 2-25
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, LimbachLK, Bruinink A and Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol. 2006, 40, 4374-4381
- Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D and Stark WJ. Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size. agglomeration, and diffusion at low concentrations. Environ Sci Technol. 2005, 39, 9370-9376
- Kwak, S. Y.; Kim, S. H.; Kim, S. S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. reparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environmental Science and Technology.2001, 35, 2388–2394.
- Borm PJ and Kreyling W. Toxicological hazards of inhaled nanoparticles — potential implications for drug delivery.J NanosciNanotechnol. 2004, 4, 521-531
- Kashiwada S. Distribution of nanoparticles in the see-through medaka (Oryziaslatipes). Environ Health Perspect. 2006, 114, 1697-1702
- Diaz-Visurraga J, Gutiérrez C, von Plessing C, Garcia A. Metal nanostructures as antibacterial agents. In: Méndez-Vilas A, Díaz J, eds. Science against microbial pathogens: communicating current research and technological advances; 2011: 210-218.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran S, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009, 8, 543-557.
- Diaz-Visurraga J, Garcia A, Cardenas G. Morphological changes induced in bacteria as evaluated by electron microscopy. In: Méndez-Vilas A, Díaz J, eds. Microscopy: Science, Technology, Applications and Education. Badajoz, Spain: Formatex. 2010, 307-315.
- Wigginton NS, De Titta A, Piccapietra F, Dobias J, Nesatyy VJ, Suter MJF, Bernier-Latmani R. Binding of silver nanoparticles to bacterial proteins depends on surface modifications and inhibits enzymatic activity. Environmental Science and Technology. 2010, 44, 2163-2168.
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles.Nanomedicine. 2007, 3, 95-101.
- Diaz-Visurraga J, Gutiérrez C, von Plessing C, Garcia A. Metal nanostructures as antibacterial agents. In: Méndez-Vilas A, Díaz J, eds. Science against microbial pathogens: communicating current research and technological advances. 2011, 210-218.
- Schuurmann, G; Markert, B. Ecotoxicology: Ecological fundamentals, chemical exposure, and biological effects. John Wiley & Sons, Inc. and SpektrumAkademischerVerlag. 1998.
- Fang J, Lyon DY, Wiesner MR, Dong J and Alvarez PJ. Effect of a fullerene water suspension on bacterial phospholipids and membrane phase behavior. Environ Sci Technol. 2007, 41, 2636-2642
- SCENIHR. The appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies [Online]. 2006.http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_003b.pdf (accessed at Aug 4, 2012).
- Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B. Ramirez, J. T. The bactericidal effect of silver nanoparticles.Nanotechnology.2005,16, 2346-2353
- Lok CN. Ho CM. Chen R. He QY. Yu WY. Sun H. Tam PKH. Chiu JF and Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006, 5, 916-924
- Amro NA, Kotra LP, Wadu-Mesthrige K, Bulychev A, Mobashery S, Liu G. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir. 2000, 16, 2789-2796.
- McQuillan J. Bacterial-Nanoparticle Interactions.Thesis for the degree of Doctor of Philosophy in Biological Sciences.University of Exeter. December 2010.
- Sondi I and Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid and Interface Science. 2004, 275, 177-182
- Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Advanced Drug Delivery Reviews. 2008, 60, 1289–1306.
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346.
- Kuo WS. Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia.Chem. Commun. 2009, 32, 4853–4855.
- Pissuwan D, Cortie CH, Valenzuela SM, Cortie MB. Functionalised gold nanoparticles for controlling pathogenic bacteria.Trends in Biotechnology. 2009, 28, 207-213.
- Burygin, G.L. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett.2009, 4, 794–801.
- Klabunde KJ, Stark J, Koper O, Mohs C, Park D, Decker S, Jiang Y, Lagadic I, Zhang D. Nanocrystals as Stoichiometric Reagents with Unique Surface Chemistry J. Phys. Chem. 1996, 100, 12142-12153.
- Huang L. Controllable preparation of Nano-MgO and investigation of its bactericidal properties.J InorgBiochem. 2005, 99, 986-993.
- Richards R, Li W, Decker S, Davidson C, Koper O, Zaikovski V, Volodin A, Rieker T, Klabunde K. Consolidation of Metal Oxide Nanocrystals. Reactive Pellets with Controllable Pore Structure That Represent a New Family of Porous, Inorganic Materials J. Am. Chem. Soc. 2000, 122, 4921-4925.
- Stoimenov PK. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002, 18, 6679-86.
- Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870.
- Thilol A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM. Cytotoxicity of CeO2 nanoparticles for Escherichia coli physico-chemical insight of the cytotoxicitymechanism.Environ. Sci. Technol. 2006, 40, 6151–6156.
- Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. lett. 2007, 90, 2139021–2139023.
- Zhang LL, Jiang YH, Ding YL, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (znonanofluids). J.Nanopart. Res. 2007, 9, 479–489.
- Jin T, Sun D, Su Y, Zhang H, Sue HJ. Antimicrobial efficacy of zinc oxide quantum dots against isteriamonocytogenes, Salmonella enteritidisand Escherichia coli O157:H7. J. Food Sci. 2009, 74, 46–52.
- Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater.2001, 3, 643–646.
- Chorianopoulos NG, Tsoukleris DS, Panagou EZ, Falaras P, Nychas G-JE. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenesbiofilm disinfection in food processing.Food Microbiology.2011, 28, 164- 170.
- Fujishima A, Honda K. Electrochemical photocatalysis of water at semiconductor electrode. Nature. 1972, 238, 27-38.
- Fujishima A, Hashimoto K, Watanabe T. TiO2 Photocatalysis: Fundamentals and Applications. Japan. BKC Inc. 1992.
- Wei C, Lin WY, Zainal Z, Williams NE, Zhu K, Kruzic AP, Smith RL, Rajeshwar K. Bactericidal activity of TiO2 photocatalyst in aqueous media: toward a solar-assisted water disinfection system. Environ. Sci. Technol. 1994, 28, 934-938.
- Pham HN, McDowell T, Wikins E. Photocatalytically-mediated disinfection of water using TiO2 as a catalyst and sporeformingBacillus pumilusas a model. J. Environ. Sci. Health A. 1995, 30, 627-636.
- Ireland JC, Klostermann P, Rice EW, Clark RM. Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl. Environ. Microbiol. 1993, 59, 1668-1670.
- Xu JF, JiW, Shen ZX, Tang SH, Ye XR, Jia DZ, Xin X Q Preparation and characterization of CuOnanocrystals. J Solid State Chem. 1999, 147, 516–519.
- Ren G, Hu D, Cheng EWC, Vargas-Reus MA, Reip P, Allaker RP. Characterisation of copper oxide nanoparticles for antimicrobial applications.International Journal of Antimicrobial Agents. 2009, 33, 587–590.
- Li B, Logan BE. Bacterial adhesion to glass and metal oxide surfaces.Colloids Surf B. 2004, 36, 81-90.
- Ravishankar, R.V., Juman, B.A. Nanoparticles and Their Potential Application as Antimicrobials. 2011
- Rupareli JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. ActaBiomaterialia. 2008, 4, 707-771.

This work is licensed under a Creative Commons Attribution 4.0 International License.





