How to Cite | Publication History | PlumX Article Matrix
Enzymatic Synthesis of Ampicillin by Immobilized Penicillin G acylase of E.coli
S.M. Atyabi1, S. Hatami Gigloo1, M. Salimi2, S.B. Momen1, A. Akbarzadeh1 and D. Nrouzian1*
1Department of Pilot Biotechnology, Pasteur Institute of Iran, Tehran 13164, Iran.
2Department of Physiology and Pharmacology, Pasteur Institute of Iran, Tehran13164, Iran.
Corresponding Author E-mail: dnsa@pasteur.ac.ir
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1050
ABSTRACT: Penicillin G acylase immobilized on to iron oxide nano particle coated with polyethyleneimine was used to synthesize b-lactam antibiotic namely ampicillin through kinetically controlled process. 6-Aminopenicillanic acid (6-APA) and R- phenylglycine methyl ester (PGME) were employed at different molar ratios to synthesis ampicillin batchwise. Lower molar ratio of b-lactam nucleus to higher molar ratio of acyl donor gave highest selectivity either in absence or presence of methanol at 35°C rather than 4°C. Both the selectivity and yields were time dependent. The immobilized enzyme was operationally stable for 6 continuous cycles.
KEYWORDS: Immobilized penicillin G acylase; Selectivity; Yield; Ampicillin.
| Copy the following to cite this article: Atyabi S. M, Gigloo S. H, Salimi M, Momen S. B, Akbarzadeh A, Nrouzian D. Enzymatic Synthesis of Ampicillin by Immobilized Penicillin G acylase of E.coli. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Atyabi S. M, Gigloo S. H, Salimi M, Momen S. B, Akbarzadeh A, Nrouzian D. Enzymatic Synthesis of Ampicillin by Immobilized Penicillin G acylase of E.coli. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10029 |
Introduction
The demands for antibiotics/semi synthetic antibiotics are high and likely the production of such drugs are ever rising (1). The industrial production of antibiotics by chemical means began in 1960s and 1970s. The production is carried out at low temperature requiring organic solvents, besides; it needs protection /deprotection of side groups along which solvents and non recyclable chemicals can be concomitantly produced (2, 3). In 1969 Cole (4) pointed out the possibility of replacing the chemical method by enzymatic synthesis, essentially employing aqueous media at physiological conditions. but due to i) synthesis at mild condition ii) stearospecificity of reaction iii) no need to protect/deprotect side chains iv) being environmentally friendly v) short routs of synthesis vi) easy down stream processing (5) enzymatic methods are preferred to chemical methods to synthesis semi synthetic antibiotic employing penicillin G acylase either in free or immobilized forms. However immobilized form of penicillin G acylase may impose diffusional restriction .This obstacle could be overcome by immobilizing penicillin G acylase onto the surface of a matrix having large surface area (6,7,). However, penicillin acylase(E.C 3.5.1.11) is an enzyme that hydrolysis penicillin into its constituent “ 6-aminopenicillanic acid and phenyl acetic acid” or transfers the acyl group from the donor( either phenylglycine methyl ester or phenylglycine) to β-lactam nucleus ( 6-aminopenicillanic acid) under kinetically or thermodynamically controlled process depending upon the nature of acyl donor. Figure 1 shows the synthesis and hydrolysis scheme of substrate (phenylglycine methyl ester) or synthesized ampicillin. The reaction takes place simultaneously i.e. PGME binds to immobilize penicillin acylase leading to the formation of an acyl –enzyme complex .This complex can either be hydrolyzed by water to yield enzyme, methanol and phenyl glycine ,or it can react with 6-APA to form ampicillin and enzyme (8) . In this article attempts are made to synthesize ampicillin through kinetically controlled process at high reactants concentration by penicillin G acylase immobilized on to iron oxide nano particles coated with polyethyleneimine (9).
Materials and Methods
Materials
Penicillin G acylase of E.coli (EC no. 3.5.1.11), polyethyleneimine, phenylglysine methyl ester (PGME), phenylglysine (PG), p-dimethylaminobenzaldehyde (p-DAB), 6-Aminopenicillanic acid (6-APA) were obtained from Sigma Chemical Company, USA. Other reagents used were of analytical grade.
Methods
Enzyme immobilization: enzyme was immobilized as reported by Atyabi et al (9)
Ampicillin synthesis
The synthesis of ampicillin was carried out in 50 ml phosphate buffer pH 6.5 containing 15 U/100 μg of immobilized enzyme, 100mM 6-APA, 300mM PGME at 35°C and 200 rpm, then pH was kept constant by the addition of 2N HCl, using Mettler pH meter. At specific time, samples were withdrawn, kept in magnetic field, passed through 0.45 μm filter and subjected to analysis by high performance liquid chromatography (HPLC). The sample was also checked for enzyme leach out from the support. One unit of synthetic activity of immobilized penicillin G acylase can be defined as the amount of immobilized enzyme that synthesizes one μ mole of ampicillin at defined temperature and pH in one minute employing 6-APA and PGME as substrates. Likewise, the operational stability was studied. After each cycle the supernatant was checked for enzyme leach out. Duration of each cycle was 2 hours and after each cycle the immobilized enzyme was washed and the washed out was checked for enzyme activity using 6-aminopenicillanic acid as a substrate.
HPLC analysis
A HPLC instrument (Wellchrom, Knauer, Germany) equipped with 4 channels K-1001 pump, an auto sampler (Triathlon Model) ,a UV detector (Model K-2600) and Chrom Gate software was used to acquire and evaluate the data . Separation was achieved employing Spherosorb ODS2 analytical column(250 mm X 4.6 mm i.d, Waters, USA).The isocratic mobile phase consisted of 0.05 M phosphate buffer pH 6.8 and methanol (60:40) which were prepared daily, degassed and passed through 0.45 μm filter. All chromatographic separations were performed at room temperature. The flow rate was set to 1 ml min-1. The wave length of the UV detector was set at λ 254 nm. The amount of reactants and product were calculated from the constructed standard curves.
Result and discussion
Synthesis of ampicillin was achieved by penicillin G acylase immobilized onto polyethyleneimine coated iron oxide nanoparticles. Therefore, ampicillin synthesis was evaluated by considering yield, productivity and selectivity(S/H).
Yield of formed ampicillin= mM of formed ampicillin/ Initial mM of 6-APA
Productivity = mM of formed ampicillin/ reaction time (h)
Selectivity= mM of ampicillin/mM of PG
High selectivity of penicillin G acylase is very important for kinetically controlled synthesis of β-lactam antibiotics. In this article attempts were made to increase the selectivity of ampicillin synthesis by employing high concentrations of substrates that reduces the water concentration in reaction mixture .This in turn could increase the probability of nucleophilic attack on acyl-immobilized enzyme complex supplied by the nucleus rather than water. Thereby, high substrates concentrations could also improve S/H ratio. The synthesis of ampicillin by immobilized penicillin G acylase was achieved at pH 6.5 (10) and 35°C. Table 1 reveals the selectivity of the reaction at high substrates concentration in kinetically controlled process in relation to time of reaction .By increasing the time of reaction selectivity increased and beyond the mentioned time the selectivity started to decrease indicating the hydrolysis of synthesized ampicillin. The highest selectivity was obtained by using 100mM 6-APA and 300 mM PGME at 35 °C, (7) obtained highest synthetic activity through the system containing cross linked aggregates of penicillin G acylase and 200 mM 6-APA:600 mM PGME . Further, to increase the selectivity, synthesis of ampicillin was performed in presence of methanol. Since water soluble solvent such as methanol could influence the equilibrium thus reducing the water molecular activity, therefore, directing the reaction towards ampicillin synthesis (11). As it can be seen from table 1, the selectivity of ampicillin synthesis has been increased when the reaction was performed in presence of methanol. The hydrolysis of product could occur beyond the appropriate time because the condensation must be determined in time since the formation of ampicillin has reached the highest, due to this reason the hydrolysis of phenylglysine methyl ester (activated acyl) and synthesized ampicillin occurred. Ferna´ndez-Lafuente, Rosell, and Guisa´n(12) studied the effect of methanol on the synthetic activity of penicillin G acylase immobilized on glyoxyl- agarose. Their studies revealed that incorporation of methanol into reaction mixture could improve the synthesis of β-lactam antibiotic using high concentration of 6-APA. Later, Illanes, Anjar , Arrieta and Aguirre( 13), studied the effect of solvents such as polyethylene glycol,glycerol and1-2 propanediol on the synthetic activity of immobilized penicillin G acylase of E. coli ( a commercial product from Roche ,Darmstadt, Germany). They found each of the above solvents could increase ampicillin yields. Figures 2 and 3 show the yields of ampicillin obtained through kinetically controlled ampicillin synthesis in presence and absence of methanol at 35°C in accordance with time. The linearity of reaction was up to 120 minutes, above 120 minutes, the hydrolysis of product was observed and led to decrease in yields and again after certain time the rise in the yields could be observed. Furthermore, the operational stability of nano penicillin G acylase was studied. It was found that the immobilized enzyme was stable over the repeated cycles of operation towards synthesis of ampicillin. In this way a nonozyme was used to synthesis ampicillin through kinetically controlled process where the selectivity of the process was towards synthesis of β-lactam antibiotic by controlling the time of reaction.
Table 1: Selectivity of ampicillin synthesized by immobilized penicillin G acylase in presence and absence of methanol at 37° C
| Time in minute | 300mM6-APA+300mM R–PGME | 300mM6-APA+100mM R–PGME | 100mM6-APA+300mM R–PGME | 300mM6-APA+300mM R–PGME +methanol | 300mM6-APA+100mM R–PGME +methanol | 100mM6-APA+300mM R–PGME + methanol |
| 10 | 0.66 | 0.53 | 0.59 | 0.93 | 0.62 | 0.84 |
| 30 | 0.84 | 0.62 | 1 | 1 | 0.73 | 1.32 |
| 60 | 0.98 | 0.75 | 1.4 | 1.14 | 0.9 | 1.9 |
| 90 | 1.18 | 0.9 | 1.7 | 1.3 | 0.9 | 2.6 |
| 120 | 1.4 | 0.85 | 2.8 | 1.5 | 1 | 5.2 |
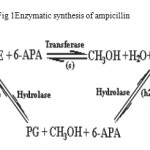 |
Figure 1: Enzymatic synthesis of ampicillin.
|
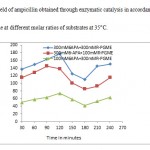 |
Figure 2: Yield of ampicillin obtained through enzymatic catalysis in accordance with time at different molar ratios of substrates at 35°C.
|
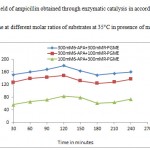 |
Figure 3: Yield of ampicillin obtained through enzymatic catalysis in accordance with time at different molar ratios of substrates at 35°C in presence of methanol.
|
Acknowledgement
The corresponding author thereby acknowledges the grant given by Iranian National Foundation for Science and Technology to carry out this research project
References
- Elander RP. Industrial production of h-lactam antibiotics. Appl Microbiol Biotechnol., 2003;61:385– 92.
- Ospina S, Barzana E, Ramirez OT, Lopez-Munguia A. Effect of pH in the synthesis of ampicillin by penicillin acylase. Enzyme Microb Technol., 1996;19:462–9.
- Youshko MI, Langen LM, Vroom E, Rantwijk F, Sheldon RA, Svedas VK. Penicillin acylase catalyzed ampicillin synthesis using a pH gradient: a new approach to optimization. Biotechnol Bioeng., 2002;78:589– 93.
- Cole M. Hydrolysis of penicillins and related compounds by the cell bound penicillin acylase of Escherichia coli. Biochem J , 1969; 115:733– 9.
- Timir B. Samanta Enzymatic synthesis of β-lactams: constrains and control. Indian Journal of Biotechnology, 2012;11:7-15.
- Andrés Illanes, José Miguel González, José Manuel Gómez, Pedro Valencia and Lorena Wilson. Diffusional restrictions in glyoxyl-agarose immobilized penicillin G acylase of different particle size and protein loading. Electronic Journal of Biotechnology, 2010; Vol.13 (.1), Issue of January 15.
- Daryoush Abedia, Mohammad Reza Fazeli and Abbas Jafarian. Optimization of enzymatic synthesis of ampicillin using cross-linked aggregates of penicillin G acylase. Iranian Journal of Pharmaceutical Research, 2004; 3: 159-164.
- Ferreira A. L. O., Giordano R. L. C., and Giordano R. C. Improving selectivity and productivity of enzymatic synthesis of ampicillin with immobilized penicillin G acylase. Brazilian Journal of Chemical Engineering, 2004; Vol. 21, (4): 519 – 529.
- Atyabi Seyed Mohammad, Akbarzadeh Azim, Salimi Mona, Momen Seyed Bahman ,Sedigeh Hatami Gigloo, Hossein Nemati and Norouzian Dariush. Optimization of penicillin G acylase immobilization process by surface response methodology using central composite design . Journal of Applied Mathematics, 2013; vol 4(1) (in Press)
- Gonc¸alves LRB, Ferna´ndez-Lafuente R, Guisa´n JM, Giordano RLC.A kinetic study of the synthesis of amoxicillin using penicillin G acylase immobilized on agarose. Appl Biochem Biotechnol., 2000;84–86:931– 45.
- Dengchao Li, Yewang Zhang, Shiwei Cheng, Qiong Gao and Dongzhi wei Enhanced enzymatic production of cephalexin at high substrate concentration with in situ product removed by complexation. Food Technology, Biotechnology, 2008; 46(4):461-466
- R. Ferna´ndez-Lafuente, C. M. Rosell, and J. M. Guisa´n. The presence of methanol exerts a strong and complex modulation of the synthesis of different antibiotics by immobilized Penicillin G acylase. Enzyme and Microbial Technology, 1998;23:305–310.
- AndréS Illanes, Soledad Anjarí,ROSA Arrieta, and Carolina Aguirre . Optimization of yield in kinetically controlled synthesis of ampicillin with immobilized penicillin acylase in organic media. Applied Biochemistry and Biotechnology, 2002; 97:165-179.

This work is licensed under a Creative Commons Attribution 4.0 International License.





