Manuscript accepted on :
Published online on: --
O. Bagheri1, N. Jonaidi Jafari1, R. Ranjbar2, S. Aminnezhad3, F. Bagheri4 and M. Saleh1,5
1Health Research Center, Baqiyatallah University of Medical science, Tehran, Iran .
2Molecular Biology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
3Microbiology Department of Islamic Azad University, Damghan, Iran.
4Islamic Azad University, Pharmaceutical Science Branch, Tehran, Iran. 5Pasteur Institute of Iran, Tehran, Iran.
Corresponding Author E-mail: baghrizohal1@yahoo.com
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1039
ABSTRACT: Bacillary dysentery caused by Shigella is one of the most important factors characterized by acute diarrhea in developed and developing countries. Probiotics reduce intensity risk of intestinal diseases. This study evaluates influences of Lactobacillus casei and Lactobacillus fermentum metabolites against Shigella Flexneri isolated from clinical samples. Antibiotic resistance of clinical samples of Shigella and Lactobacillus casei and Lactobacillus fermentum were assessed using agar-based minimal inhibitory concentration assay. 34 clinical samples of Shigella flexneri from patients were assayed for the antimicrobial activity of Lactobacillus metabolites using broth micro dilution method, minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). Standard bacterial growth curves were drawn up by performance of turbidometric measurements to compare antibacterial effects of Lactobacilli metabolites against clinical samples. Antibiogram was carried out for 34 samples with positive culture. Antibiotic resistant bacteria were revealed for tetracycline, streptomycin and tobramycin. In this study chloramphenicol, tobramycin and amikacin resistance in Lactobacillus casei were detected and Lactobacillus fermentum was resistant to ceftazidime, tobramycin and amikacin. MIC and MBC of Lactobacillus casei against Shigella flexneri was 62.5 and 62.5. MIC and MBC of Lactobacillus fermentum against Shigella flexneri were 62.5, 15.6 respectively. It shows that Lactobacillus casei and Lactobacillus fermentum can be used as probiotic candidate against Shigella flexneri.
KEYWORDS:
Lactobacillus casei; Lactobacillus fermentum; Shigella flexneri; probiotic; antibiogram.
| Copy the following to cite this article: Bagheri O, Jafari N. J, Ranjbar R, Aminnezhad S, Bagheri F, Saleh M. Evaluation of Antimicrobial Activity of Lactobacillus casei and Lactobacillus fermentum Metabolites on the Shigella flexneri Clinical Samples. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Bagheri O, Jafari N. J, Ranjbar R, Aminnezhad S, Bagheri F, Saleh M. Evaluation of Antimicrobial Activity of Lactobacillus casei and Lactobacillus fermentum Metabolites on the Shigella flexneri Clinical Samples. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9958 |
Introduction
Shigella belongs to the order Enterobacteriaceae, species of which are normal inhabitants of the human intestinal tract and can cause dysentery. Shigella species are classified by four serogroups, S.dysenteriae, S.flexneri, S.boydii and S.sonnei(1). Significant amounts of mucus and blood accompanied by diarrhea, abdominal pain, rectal pain, fever and tenderness of upper abdominal are clinical features of infection with inflammatory, invasive diarrhea caused by Shigella(1-3). Shigellosis is a self-limited disease(4), but the results in international research centers showed that 7% of patients afflicted with fatal infection(3). Shigellosis is highly contagious and a single patient can infect 10 or more people in a year while less than 200 Shigella organisms are required for aninfection(5). It can be spread in communities with low health standard. This characteristic and several other factors raised Shigella as military health risks(6).
Many different ways of contamination and using antibiotics to treat shigellosis can actually make the germs more resistant in the future(2). Widespread overuse and inappropriate use of antibiotics leads to drug-resistant bacteria therefore provision and codification of a protocol for prevention and treatment are mandatory under natural patterns(7). Application of antibiotics for treatment and prevention not only develop drug resistance(8) but disturb the indigenous and natural microflora of the gastrointestinal tract, consequently the body exposed to different intestinal diseases like diarrhea(9). On the contrary oral probiotics as microorganism can suppress the microbial pathogen and can help regulate and boost the immune system(10). Probiotic organisms are live microorganisms commonly consumed as part of fermented foods or as dietary supplements that inhibit the growth of harmful bacteria and play the role of prevention in different parts of the body included gastrointestinal system and urinary- reproductive system(11-12).
Different strains of Lactobacillus like lactobacillus casei and lactobacillus fermentum were found to be very active. Some of useful effects and roles of probiotics are: improving the balance of flora and promoting good digestion in the gastrointestinal tract, making good health and vitality of urogenital system, boosting immune function, inhibiting pathogens and toxin producing bacteria and improving the nutritional value of fermented foods (13-15).
The antagonistic interactions between some bacterial strains producing lactic acids were reported initially by Pasteur and Joubert in 1908. They used them in treatment of gastrointestinal tract. It has been shown that the most effective factor in the mechanism(s) of the antibacterial activity of probiotic Lactobacillus strains appears to be lowering the pH by producing lactic acid and antibacterial compounds and its effect on protein expression to survive passage through the gastrointestinal tract(16-17).
Nowadays Lactic acid bacteria such as Lactobacillus casei and lactobacillus fermentum have been shown to be effective in the treatment and prevention of acute diarrhea with lowest side effects(17).
However, the primary and secondary antimicrobial metabolites produced in different stages of bacterial growth are considered as an effective agent to inhibit growth of putrefactive, corrosive, toxic and pathogenic agents(18).
The rise in antibiotic resistance and overuse of antibiotics reduce the intestinal normal flora by replacing pathogens such as Shigella, consequently increase the probability of infection. According to resistance of bacteria to different antibiotics and also in order to decreasing antibiotic uptake it is necessary to use alternative compounds(19). Probiotics produce numerous metabolites that can be a good candidate for this purpose. The purpose of this study is that using these probiotics as an alternative to antibiotics will lie in the prevention of damage to the intestinal microflora following antibiotics and also can kill harmful and pathogenic bacteria in intestine. It plays a significant role in enhancing immune system against pathogens(20).
This study was carried out to detect and evaluate antimicrobial activity of Lactobacillus casei and Lactobacillus fermentum as probiotics against microorganisms producing bacillary diarrhea, Shigella flexneri(as agent of bioterrorist attack). It is worth mentioning that Lactobacillus casei and Lactobacillus fermentum act as probiotic agents in antimicrobial function.
Materials and Methods
Lactobacillus casei and Lactobacillus fermentum preparation, biochemical aspects of the culture and preparation of Lactobacillus metabolites
Lactobacillus casei (PTCC 1608) and Lactobacillus fermentum (PTCC 1638) used in the present study were obtained from Iran laboratory reference center of Iranian Research Organization for Science. These strains were transferred to research laboratory and inoculated in MRS Broth media (Deman-Rogosa-Sharpe Broth) (30912 Fluka, Sigma-Aldrich GmbH, US). These media were incubated for 48 hours at 37°C in the anaerobic jar containing a gas pack generating an atmosphere of 5% CO2. Identification of Lactobacillus Bacteria using biochemical tests and gram stain test was performed.
The metabolites produced by bacteria were isolated from the supernatant fluid by centrifuging at 15000×g. The supernatant was passed through a 45 µm filter and serial dilutions for bacteria testing were prepared by sterile water.
Shigella flexneri preparation, biochemical and serological characteristics of the culture
Sampling was taken from suspicious patient at hospital and laboratory by specialist. Collected samples transferred to laboratory in appropriate media, then cultured on XLD medium (Merck, Germany). Then the suspicious colonies transferred to TSI media (Merck, Germany). In addition to proper colony isolation, both biochemical testing such as Urea and Motility testing and serological testing like monovalent Shigella antisera (Bahar-Afshan Company, Iran) must be performed to properly identify Shigella strains.
Antibiogram test of Lactobacillus casei and Lactobacillus fermentum, Shigella flexneri
For Lactobacillus casei, Lactobacillus fermentum and 30 clinical samples of Shigella antibiogram test using the disc diffusion methodology carried out by following antibiotics: Streptomycin, Co–amoxiclave, Nalidixic acid, Ceftazidime, Tetracycline, Tobramycin, Ticarciliin, Cefotaxime, Ceftriaxone (Padtan Teb Company, Iran)(21).
Agar Well Plate Method
A well was made in each 34 plates with sterile borer (5mm).Lactobacillus casei and Lactobacillus fermentum metabolite (100 µl) was introduced into the well and then a 0.5 McFarland suspension of Shigella(80 µl) poured on the surface of the plates. The petri dishes were incubated at 37°C for 48 hrs. All samples were tested in triplicates. Microbial growth was determined by measuring the diameter of zone of inhibition in millimeters(22).
Determination of MIC and MBC of the metabolites for Lactobacillus casei
In this method, round-bottom wells of 96-well plates with a volume of 300 were used. A range of concentrations of Lactobacillus from 0.12 µl/ml to 250 µl/ml were prepared using medium Mueller Hinton Broth (Merck, Germany). 200 µl of different concentrations and then 100 µl of the bacterial suspension were added to each well. The final concentration of bacteria in each well was (0.5-1)×106 CFU/ ml.
The plates were incubated at 37°C for 24 hours. Clinical and Laboratory Standards Institute (CLSI) has suggested methods to determine the susceptibility of microbes(23). MIC values were detected by ELISA reader. Furthermore the conventional colony count method for determining minimal bactericidal concentration (MBC) was performed. The results were drawn up on the curve.
Microbiological Turbidimetric Method for the Assay of Lactobacillus casei
One milliliter of Lactobacillus casei metabolite exposed to 500 milliliter of 1 McFarland suspension of each clinical samples individually. The wavelength at which turbidity was measured to achieve maximum detection were 650 nm, over 0, 2, 4, 6, 12, 20 & 24 hrs exposure time. The results of 30 samples were drawn up on the curve.
Statistical Analysis
The data and results were analyzed using chi – square and Fisher with SPSS software version 17. All data were expressed as mean. For comparison of quantitative data mean between groups ANOVA test (one-way analysis of variance) and Tukey were used. A value of P<0.05 was considered statistically significant for all tests.
Results and Discussion
Detection of Shigella flexneri phenotypes
From clinical samples of the patients with acute diarrhea, 34 samples were separated. Shigella flaxneri were diagnosed by serological and biochemical tests.
Antibiogram Test Results of Lactobacillus casei and Lactobacillus fermentum and 34 Clinical Samples Shigella flexneri
The antibiograms of 34 cases of positive cultures related to Shigella flaxneri illustrated 97.06% of resistance to Tetracycline, 91.17% of resistance to Streptomycin, and 17.65% of resistance to Tobramycin. Significant level was considered as a value of P<0.05. The results have been shown in table 1.
Table 1: Antibiogram Test of Shigella flexneri separated from Clinical Samples.
| Abb. Of Antibiotic | S | AMC | NA | CAZ | TE | TOB | TIC | CTX | CRO |
| Number of antibiotic sensitivity strain | 3 | 14 | 21 | 32 | 1 | 28 | 31 | 30 | 31 |
| Number of Intermediate strain | 0 | 10 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Number of antibiotic resistance strain | 31 | 10 | 13 | 1 | 33 | 6 | 3 | 3 | 3 |
| Antibiotic sensitivity% | 8.83 | 41.17 | 61.76 | 94.12 | 2.94 | 82.35 | 91.17 | 88.23 | 91.17 |
| Intermediate strain% | 0 | 29.41 | 0 | 2.94 | 0 | 0 | 0 | 2.94 | 0 |
| antibiotic resistance% | 91.17 | 29.42 | 38.24 | 2.94 | 97.06 | 17.65 | 8.83 | 8.83 | 8.83 |
A value of P<0.05 was considered statistically significant. (S: Streptomycin, AMC: Co-amoxiclave, NA: Nalidixic Acid, CAZ: Ceftazidime, TE: Tetracycline, TOB: Tobramycin, TIC: Ticarciliin, CTX: Cefotaxime, CRO: Ceftriaxone)
Table 2: Antimicrobial activity disk diffusion method for two strains of Lactobacillus.
|
Antibiotic
|
chloramphenicol | Tobramycin | Amikacin | Ceftazidime | ceftriaxone |
| Abb. Of Antibiotic name | C | TOB | AN | CAZ | CRO |
| Lactobacillus casei | R | R | R | S | S |
| Lactobacillus fermentum | S | R | R | R | S |
Antibiogram test by disk diffusion method has been done for Lactobacillus casei and Lactobacillus fermentum. The results have been presented in table 2.
The Results of Antimicrobial Activity of Lactobacillus casei against Shigella flexneri
The Results of Well plate method
Agar Well Plate Method demonstrated that 94.20% of the samples were sensitive to Lactobacillus casei (Fig.1-A) and Lactobacillus fermentum (Fig.1-B) and inhibition of the bacterial growth were measured in the average of 19 mm. Only two samples of Shigella flexneri were resistance to Lactobacilli. According to the reports, produced zones of bacterial growth-inhibition were not appeared in the first 24 hrs of incubation time. Diameter of inhibition zone was recorded and the results were analyzed by SPSS software version 17. There is statistically significant difference between the results) P<0.05. To determine the MBC, of the wells that were not blurred, positive and negative control samples were cultured on the NB Media. After 24 hours MBC was investigated based on minimum antimicrobial concentration that prevents the growth of bacteria on the surface of the plate(24).
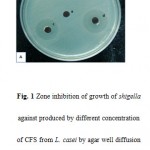 |
Figure 1: Zone inhibition of growth of shigella against produced by different concentration of CFS from L. casei by agar well diffusion method. a: MRS(100 μl/ml), b: CFS(10 μl/ml),c:CFS(50 μl/ml), d: CFS(100 μl/ml). |
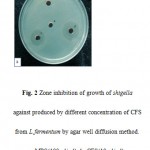 |
Figure 2 Zone inhibition of growth of shigella against produced by different concentration of CFS from L.fermentum by agar well diffusion method. a: MRS(100 μl/ml), b: CFS(10 μl/ml),c:CFS(50 μl/ml), d: CFS(100 μl/ml). |
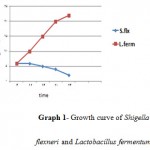 |
Graph 1: Growth curve of Shigella flexneri and Lactobacillus fermentum.
|
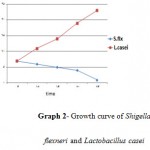 |
Graph 2: Growth curve of Shigella flexneri and Lactobacillus casei.
|
The Results of Microbiological Turbidimetric Method
According to the Standard bacterial growth curve in the control group, the optimal conditions for the growth of Lactobacillus fermentum, Lactobacillus casei and clinical samples of shigella were observed in the first day. In the second group, exposure of Lactobacillus metabolites to the clinical samples of Shigella flexneri had been found a highly significant decrease in the bacterial growth (curve1-1& curve1-2).
The Results of MIC and MBC of Lactobacillus casei and Lactobacillus fermentum Metabolites
The results for the minimum inhibitory concentration (MIC) and minimum bacteriostatic concentration (MBC) of Lactobacillus casei against Shigella flexneri using micro dilution method was 62.52 and 62.52 respectively.
MIC and MBC of Lactobacillus fermentum against Shigella flexneri were 62.52 and 15.67 respectively. A value of P<0.05 was considered statistically significant.
In Microdilution method MIC values were analyzed, both visually and by enzyme-linked immunosorbent assay reader (TECAN, Sunrise). The results were consistent and reliable to each other.
Probiotic organisms are live microorganisms thought to be used in treatment and prevention of some infectious diseases. Probiotics serve as a barrier for the colonization of pathogens to prevent disease, spread and invasion of bacteria in the organs which can be settled (8, 3). Several studies showed that Lactobacilli have positive role in treating and preventing of gastrointestinal system disorders. Lactobacilli are able to be connected to human and animal epithelial cells in gastrointestinal system(10). Toba et.al tested culture supernatant fluids of different Lactobacilli against pathogenic bacteria like Listeria monocytogenes, Salmonella and Staphylococcus aureus. Turbidity was monitored in different times and a rapid decline in cell number was recorded.
In this study metabolites of Lactobacillus casei and Lactobacillus fermentum were tested against pathogenic strains obtaining from clinical samples and turbidity measurements determined that the metabolites prevent bacterial growth and decline its turbidity considerably.
Hirano et al have studied on the antibacterial activity detected in supernatant fluid of Lactobacillus plantarum, Lactobacillus sakei and Lactobacillus curvatusin plate by WDA method. Inhibition zones of these substances presented inhibition effects on a vast range of pathogen bacteria like Yersinia enterocolitica and Listeria.
In present study Lactobacillus casei and Lactobacillus fermentum showed antimicrobial activity against Shigella flexneri in clinical samples and Lactobacilli metabolites against human pathogenic bacteria displayed notable and significant size of the zone of inhibition.
According to the results of this study, it is obvious that Lactobacillus casei and Lactobacillus fermentum can inhibit Shigella flexneri growth as human pathogenic agent and it can be a good candidate as a probiotic factor against invasive, inflammatory and toxigenic diarrhea. It also recommended that metabolites from Lactobacillus casei culture and cell body can be used against pathogenic bacteria including intestinal pathogens like Shigella flexneri and its products are active in hard temperature conditions and common antibiotics, its antibacterial activities remain constant and stable.
Acknowledgment
The authors acknowledge Dr.Reza Ranjbar, dean of Molecular Biology Research Center of Baghiatalah University for sharing the clinical samples and facilities of his laboratories.
References
- Ranjbar, R., Dallal, M.M.S., Pourshafie, M.R., Epidemiology of Shigellosis With Special Reference to Hospital Distribution of Shigella Strains in Tehran. IJCID- Iranian Journal of Clinical Infectious Diseases, 2008; 3(1): 35-38.
- Hurley, B., Thorpe, C., Acheson, D., Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun., 2001; 69(10): 6148-55.
- Ranjbar, R., Hosseini, M., Kaffashian, A., Farshad, S., An Outbreak of Shigellosis Due to Shigella Flexneri Serotype 3a in a Prison in Iran. Arch. Iran. Med., 2010; 13: 413-416.
- Ranjbar, R., Soltan Dallal, M.M., Talebi, M., Pourshafie, M.R., Increased isolation and characterization of Shigella sonnei obtained from hospitalized children in Tehran, Iran. J. Health Popul. Nutr., 2008; 26(4): 426-30.
- Russell, R., Oral infections. In Sussman, M., ed. Molecular Medical Microbiology, University of Newcastle Upon Tyne, The Medical School, U.K., 2002; 2: 1065-1079.
- Kale-Pradhan, P., HKJassal, Wilhelm, S., Role of Lactobacillus in the prevention of antibiotic-associated diarrhea: a meta-analysis. Pharmacotherapy, 2010 Feb; 30(2): 119-26.
- Max Sherman, R.P., Sherman Consulting Services, Inc. Warsaw, Indiana, US. Probiotics and Microflora. Pharm. 2009; 34(12): 42-44.
- Famularo, G., Pieluigi, M., Coccia, R., Mastroiacovo, P., Simone, C.D., Microecology, bacterial vaginosis and pro-biotics: perspectives for bacteriotherapy. Medical Hypotheses, 2001; 56: 421-430.
- Krieg, N., Ludwig, W., Whitman, W.B., Hedlund, B.P., Paster, B., Staley, J., Ward, N., Brown, D., Parte, A.(ed): Bergey’s Manual of Systematic Bacteriology. 2nd edn. Originally published by Williams & Wilkins, 2010 Nov; pp 951-973.
- Gorbach, S., Probiotics and gastrointestinal health. Am. J. Gastroenterol., 2000 Jan; 95 (1): S2-4.
- Duerden, B., Collier, L., Balows, A., Sussman, M., Topley & Wilson’s Microbiology & Microbial Infections: Bacterial Infections, Enterobacteriaceae, 10th edn. New york, USA, 2005; pp 491-505.
- Sreekumar, O., Hosono, A., Immediate effect of Lactobacillus acidophilus on the intestinal flora and fecal enzymes of rats and the in vitro inhibition of E coli I coculture. J. Dairy Sci., 1990; 83(5): 931-9.
- Reid, G., Jass, J., Sebulsky, M., McCormick, J., Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev., 2003; 16: 658-672.
- Thoreux, K., Schmucker, D.L., Kefir Milk Enhances Intestinal Immunity in Young but Not Old Rats. J. Nutr., 2001 March; 131(3): 807-812.
- Kimoto-Nira, H., Ohmomo, S., Nomura, M., Kobayashi, M., Mizumahi, K., Okamoto, T., Interference of in vitro and in vivo growth of several intestinal bacteria by Lactococcus strains. J. Microbiol. Biotechnol., 2008 Jul; 18(7): 1286-9.
- Zhu, W., Liu, W., Wu, D., Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J. Appl. Microbiol., 2000 May; 88(5): 877-86.
- Ennahar, S., Deschamps, N., Anti-Listeria effect of enterocin A, produced by cheese-isolated Enterococcus faecium EFM01, relative to other bacteriocins from lactic acid bacteria. J. Appl. Microbiol., 2000 Mar; 88(3): 449-57.
- Ferna´ndez, M., Boris, S., Barbe´s, C., Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. Journal of Applied Microbiology, 2003; 94: 449-455.
- Lu, L., Walker, W.A., Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am. J. Clin. Nutr., 2001 June; 73(6): 1124S-1130S.
- Madden, J.A.J., Hunter, J.O., A review of the role of the gut microflora in irritable bowel syndrome and the effects of probiotics. British Journal of Nutrition, 2002; 88, Suppl. 1: S67-S72.
- Bauer, A.W., Kirby, M.M., Sherris, J.C., Tuurck, M., Antimicrobial susceptibility testing by a standardised single disc method. Am. J. Clin. Path., 1966 Apr; 45(4):493–496.
- Saeidnia, S., Yassa, N., Rezaeipoor, R., Shafiee, A., Comparative investigation of the essential oils of Achillea talagonica Boiss. and A. millefolium, chemical composition and immunological studies. Journal of Essential Oil Research, 2004; 16(3): 262-265.
- Wikler, M.A. (ed): Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd Edition edn. Clinical and Laboratory Standards Institute, 2009 Jan; pp 1-6.
- Murray, P. R., Baron E. J., Jorgensen J. H., Pfaller M. A., and Yolken R. H., (eds): Manual of Clinical Microbiology, 8th edn. Washington, DC: ASM Press, 2003; pp 1037-1212.

This work is licensed under a Creative Commons Attribution 4.0 International License.





