How to Cite | Publication History | PlumX Article Matrix
Fungal Hydrolysis and Saccharification of Rice Straw and Ethanol Production
H.R. Madian1, Nour Sh. EL-Gendy1*, Laila A. Farahat1, Mervate A. Abo-State2 and Ahmed M.E. Ragab3
1Egyptian Petroleum Research Institute (EPRI), Nasr City, Cairo, Egypt.
2National Center for Radiation Research and Technology (NCRRT), Nasr City, Cairo, Egypt
3Faculty of Science (Girls Branch), Al-Azhar University, Cairo, Egypt.
Corresponding Author E-mail: nourepri@yahoo.com
DOI : http://dx.doi.org/ http://dx.doi.org/10.13005/bbra/1025
ABSTRACT:
KEYWORDS:
| Copy the following to cite this article: Madian H. R, EL-Gendy N. S, Farahat L. A, Abo-State M. A, Ragab A. M. E. Fungal Hydrolysis and Saccharification of Rice Straw and Ethanol Production. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Madian H. R, EL-Gendy N. S, Farahat L. A, Abo-State M. A, Ragab A. M. E. Fungal Hydrolysis and Saccharification of Rice Straw and Ethanol Production. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9892 |
Introduction
Egypt is facing a high population growth rate, which demands for an increase in agricultural production efficiency. Consequently, agricultural field residues will increase. Crop residues in Egypt, have been estimated to amount range from 30-35 million tons/year, e.g. rice husk 1.6, rice straw 3.6, wheat straw 6.9, corn stover 3.3, cotton stalks 1.6, sugar-cane residue 6.8, sugar beet residues 0.32 million tons/year (Abou Hussein and Sawan, 2010). At present, these waste streams are not economically re-used; create air, surface and water pollution and having therefore a negative impact on economy and environment. A clear example is the severe air pollution, largely attributed to uncontrolled burning of solid waste and agriculture residue. It mainly affects Greater Cairo GC, which hosts 20% of the nation’s population. Open burning of solid waste and agriculture residue alone has been estimated to account for 42% of annual air pollution. Air pollution in GC peaks every year in the autumn season, creating the acute air pollution episode known as the black cloud period. Tod, one of the main anthropogenic causes of the Black Cloud phenomenon is suspected to be the uncontrolled open-burning of rice straw (El-Dorghamy, 2007). This comes at a time where Egypt is rapidly approaching energy dependency. Utilizing this “waste” as a resource, or fuel, for bioenergy systems would entail many environmental and developmental benefits.
Ethanol is nowad an important product in the fuel market. Its market is expected to reach 100 billion liters in 2015 (Harmsen et al., 2010). Recently, efforts have increased worldwide towards the commercial production of ethanol and alternative fuels, especially liquid transportation fuels. The development of second generation bio-ethanol made from ligno-cellulosic biomass can increase the sustainability of feedstock production without competing with food production or the cultivation of farmland and from the waste-management stand-point, producing bio-ethanol from agricultural wastes is environmentally beneficial. Bio-ethanol from ligno-cellulosic biomass is one of the most employed liquid biofuels due to the easy adaptability of this fuel to existing engines and because this is a cleaner fuel with higher octane rating than gasoline (Sukumaran et al., 2010).
There are three main steps involve in the conversion of rice straw to bioethanol (i) pretreatment and hydrolysis, (ii) enzymatic saccharification of cellulose and hemicelluloses, and (iii) ethanol fermentation. The aim of pretreatment is the disruption of the ligno-cellulose structure, improving cellulose and hemicelluloses accessibility to produce reducing sugars for fermentation to ethanol. Nowad, steam explosion, which requires high pressures and temperatures and acid hydrolysis which is corrosive, are the most widely used pretreatments although the severity of these processes generates by-products that affect the adversely subsequent steps (Alvira et al., 2010). An alternative to avoid these problems is the use of biological pretreatments and hydrolysis, which present additional advantages as being cheaper, safer, less energy-consuming and more environmentally friendly. Cellulases are relatively costly enzymes, and a significant reduction in cost will be important for their commercial use in the preparation of cellulosic feedstock. For economic point of view, there is a need to increase cellulase enzyme volumetric productivity by using cheap and readily available substrates. Azzaz et al. (2012) reported that cellulase with its immense importance is being imported for use in Egypt at a high cost. So the local production of such enzymes may reduce the cost of importation and encourage self-reliance
Therefore, this work aims to investigate the efficiency of two locally isolated fungal strains Trichoderma viride F-94 and Aspergillus terreus F-98 to utilize Egyptian rice straw as an inexpensive (100 Egyptian pounds/ton) and readily available substrate for cellulase production throughout solid state fermentation SSF processes. This work is also expected to provide useful information for assessing the feasibility of using two-stage process, separate hydrolysis and fermentation SHF involving sequential hydrolysis and saccharification of rice straw using F-94 and F-98 and then sugars fermentation for bioethanol production by two locally isolated yeast strains Candida tropicalis Y-26 and Saccharomyces cerevisiae Y-39.
Material and methods
Media
Media used for maintenance and culture preparation of filamentous fungi and yeast were; potato dextrose (PD) agar (Oxoid, 1982) and Wickersham’s (WH) agar (Wickerham, 1951) media, respectively.
Microbial strains and inoculum preparation
The two fungal strains; Trichoderma viride F-94 and Aspergillus terreus F-98 used for solid state fermentation SSF of rice straw in this study were previously isolated for their ability to degrade different lignocellulosic wastes (Abo-State et al., 2012). Spore suspension, inoculum preparation and substrate inoculation were carried out as described by (Abo-State et al., 2003).
The two yeast strains Candida tropicalis Y-26 and Saccharomyces cerevisiae Y-39 used for hydrolyzates fermentation to bioethanol were previously isolated for their ability to utilize different saccharides as sole carbon and energy sources (Abo-State et al., 2012). Active cultures for inoculation were prepared by growing the organisms in WH medium for 48 h at 30oC in a shaking incubator of 150 rpm.
Analytical Methods
Hemi-cellulose, cellulose, and lignin percent in rice straw samples before and after SSF were determined in Agricultural Research Center, Giza, Egypt. The mono-saccharides were determined and quantified before and after fermentation of hydrolyzate at National Research Center, Giza, Egypt, according to the method reported by Askar et al. (2009), by High Performance Liquid Chromatography (HPLC) equipped with 10A Shimadzu Shim-pack SCR-101N column (7.9 mm x 30 cm), using de-ionized water as the mobile phase (flow rate 0.5 mL / min at 40oC) and refractive index detector. The injected volume was 20 μL. Sugar standards were used for quantification of different sugars (glucose, xylose, arabinose and rhamnose) in the samples. Ethanol production was analyzed by Gas chromatography (model 6890, Agilent), equipped with flame ionization detector and nominal capillary column (60 m x 530 µm x 5.00 µm). Helium was the carrier gas, flow rate was 25 mL/min. Oven and detector temperature was 300oC.Total reducing sugars (TRS) were determined by 3, 5-dinitro salicylic acid (DNS) method (Miller, 1959) and glucose was used as a standard. The samples were stored in a fridge at -18oC until analysis to prevent spoilage by microbes and loss of ethanol. All experiments were carried out in triplicates.
Substrate preparation
Rice straw was collected from local agricultural field, air-dried then chopped, ground and sieved to size (0.5-1cm). Ground materials were then stored in plastic bags at room temperature until analysis and treatment. Rice Straw was initially analyzed for determination of hemi-cellulose, cellulose and lignin content (31.22, 34.80 and 10.18% (%wt.), respectively).
Enzyme production and extraction
Solid state fermentation SSF was carried out for enzyme production using each fungal strain individually. About 50 g of ground rice straw was moistened with 250 mL of moistening agent (15 g/L yeast extract, 10 g/L glucose, 2.5 g/L NH4Cl, 2.0 g/L K2HPO4, 0.5 g/L MgSO4.7H2O, 0.1 g/L CaCl2 and 0.5 g/L KCl) and then steam-pretreated by autoclaving at 121oC and 1.5 bars for 20 min after that the media was inoculated with 10 mL spore suspension (107 spores/mL) and incubated under static condition at 30oC for 7 d. Crude enzymes from fermented mash were extracted by adding 10 fold of distilled water (1:10 solid to liquid ratio), mixed well and placed on shaker with the agitation speed of 150 rpm for 2 h. After complete mixing it was filtered through cheese cloth and the residues were discarded and the filtrate was centrifuged at 10,000 rpm for 20 min to get the supernatant (crude enzymes) free of spores.
Estimation of enzyme activities
Endo-glucanase (carboxymethyl cellulase CMCase) activity in the culture filtrate was determined by incubating 0.5 mL of crude enzyme sample with 0.5 mL of 1% carboxymethyl cellulose (0.05 M citrate buffer pH 5) at 50oC for 30 min. After incubation, the reaction was stopped by the addition of 1.5 mL DNS and then boiled for 10 min in boiling water bath. The reaction mixture was allowed to cool and the TRS were estimated by DNS method.
For the estimation of exo-glucanase (filter paper FPase) activity, 0.5 mL of culture filtrate was added to test tube containing 25 mg of Whatman No.1 filter paper and 0.5 mL of 0.01 M citrate buffer pH 4.8, incubated at 50oC for 30 min. After that, the reaction was stopped by the addition of 1.5 mL DNS and then boiled for 10 min in boiling water bath. The reaction mixture was allowed to cool and the TRS were estimated by DNS method.
One unit of enzyme activity is defined as the amount of release 1 µmol of reducing sugars equivalent to glucose under the assay conditions.
Biological hydrolysis and saccharification of rice straw
Enzymatic hydrolysis and saccharification EHS
Ten g of chipped and ground rice straw were put into 250 mL Erlenmeyer flask. The flasks were then steam-pretreated by autoclaving at 121oC and 1.5 bars for 20 min. The crude enzymes of each fungal strain were used individually in a ratio of 1:14 (w:v, substrate:enzyme). The flasks were then incubated in a shaking water bath at 50oC and 150 rpm for 48 h. The hydrolysis reaction was terminated by heating at 80oC for 5 min, then centrifuged at 10,000 rpm for 20 min and the TRS were estimated by DNS method.
Hydrolysis and saccharification by whole fungal cells in presence of nutrients through SSF process
Ten g of chipped and ground rice straw, moistened with 25 mL nutrient solution (0.5 g/L MgSO4.7H2O, 0.5 g/L CaCl2.H2O, 3 g/L KH2PO4 and 10g/L (NH4)2SO4) were put into 250 mL Erlenmeyer flask. The flasks were then steam-pretreated by autoclaving at 121oC and 1.5 bars for 20 min, inoculated with 10 mL spore suspension of each fugal strain individually (107 spores/mL) and incubated under static condition at 30oC for 7 d. The solid material was then mixed vigorously with 100 mL distilled water for extraction of soluble reducing sugars, then filtered with cheese cloth to separate the contents into liquid and solid parts and the TRS were estimated by DNS method.
Hydrolysis and saccharification by whole fungal cells in absence of nutrients through SSF process
Same steps were carried out as listed above but rice straw was moistened with 25 mL distilled water instead of nutrients solution.
The percent saccharification was calculated as described by Uma et al. (2010) by the following formula:
![]()
Bioethanol Fermentation
This was carried out according to Yu and Zhang (2004), where peptone (10.0 g/L), KH2PO4 (2.0 g/L), MgSO4.7H2O (1.0 g/L) were added to the hydrolyzate obtained from saccharification by whole fungal cells in absence of nutrients through SSF process and then sterilized by autoclaving at 121oC for 20 min. The hydrolyzate was then individually inoculated with 10 % (v/v) yeast suspensions. The inoculated hydrolyzates were incubated at 30oC for 48 h at 150 rpm. After incubation, the fermented hydrolyzates were centrifuged at 10000 rpm for 10 min. The produced ethanol and residual TRS concentrations were determined. The ethanol yield was calculated by the modified formula (2) proposed by Gunasekaran and Kamini (1991).
![]()
Results and discussion
Activity of crude enzymes
The two fungal strains T.viride F-98 and A. terrus F-94 expressed good yields of cellulases CMCase and FPase from rice straw but with different efficiencies. Endo-glucanase (CMCase) production was significantly high by the two fungal isolates compared to that of exo-glucanase (FPase). T. viride F-94 expressed lower cellulases’ production on rice straw than that of A. terreus F-98, recording; CMCase of ≈ 161 and 232 IU/mL, and FPase of ≈ 76 and 80 IU/mL, respectively.
Fungi that have been reported to produce cellulases include P. chrysosporium, Trichoderma, Aspergillus, Schizophyllum and Penicillium. Among all of these fungal genera, Trichoderma and Aspergillus have been most extensively used for cellulase production (Helal, 2006; Chandra et al., 2007; Irfan et al., 2011; Azzaz et al., 2012).
Raimbault (1998) and Pandey et al. (2000) reported that the major bottleneck in biomass to ethanol conversion is the cost of cellulase enzymes and any strategy which can bring down the production cost of cellulases can significantly reduce the cost of bio-ethanol. Solid state fermentation SSF is a well established technology for enzyme production and provides several advantages like lower cost of operation, lesser infrastructure requirements, ability to operate with less skilled manpower and above all, the ability to use cheap agro-industrial residues and biomass as raw materials (Yang et al., 2004, Singhania et al., 2009).
Production of cellulases by fungi throughout SSF technique using agriculture wastes has been previously reported (Umikalsom et al., 1997; Dogaris et al., 2006; Gao et al., 2008; Abo- state et al., 2010; Fawzi and Hamdy, 2011). Kang et al. (2004) have reported the production of 129 units of CMCase in SSF of rice straw by A. niger KK2. Gao et al. (2008) reported that Aspergillus M11, when grown on lignocellulosic materials in SSF produced 581 U/g CMCase and 243 U/g FPase. Kocher et al. (2008) reported endo-glucanase CMCae activity of 0.12 IU/mL and exo-glucanase FPase activity of 0.09 IU/mL, using rice straw inoculated with Trichoderma harzianum Rut-8230. Abd El-Zaher and Fadel (2010) reported that T. reesei in SSF can secrete a complex array of degradative enzymes for lignocellulosic wastes. Abo-State et al. (2010) reported that Aspergillus MAM-23 produced CMCase 309 U/mL on rice straw in SSF process. Jahromi et al. (2011), reported production of cellulase enzymes FPase 480.48 U/gDM and CMCase 363.72 U/gDM by A. terreus ATCC-74135 in SSF using rice straw as a substrate.
According to Chandra et al. (2007) and Gao et al. (2008); the comparisons of cellulase activities produced by different researches are not readily made in quantitative manner as no standard conditions of cellulase activity assay have been adopted. Also the difficulty in comparison between cellulase(s) activities depends on the difference between strains used in production, materials as solid matrix, condition of production; submerged Sm or solid state SS fermentation, determination assay and other physical factors.
Different process for hydrolysis and saccharification of rice straw
The TRS yields are criterion to the sugars liberated during hydrolysis. The ground rice straw produced the lowest yield of TRS ≈ 1 g/L with saccharification% of ≈ 4%. Ground and steam treated (autoclaved) rice straw, showed higher yield of TRS and saccharification% of ≈ 6 g/L and 16%, respectively.
Helal (2006) reported that; physical pretreatment before fungal treatment increases the surface area of the cellulose by reducing particle size. Sher et al. (2010), reported that the steamed lignocellulosic waste would have loosed the shield formed by lignin and hemi-cellulose which makes the substrate more acceptable for fungal strains and increase cellulose digestibility.
Enzymatic hydrolysis and saccharification of rice straw
According to Lo et al. (2009); enzymatic hydrolysis of cellulosic feedstock has several advantages over chemical processes because of its high saccharification efficiency, less energy consumption and avoidance of pollution.
It is obvious from TRS yields illustrated in Figure.1, that; hydrolysis and saccharification efficiencies were in parallel with the amount and activity of the produced crude cellulases. Enzymatic saccharification of rice straw by T. viridie F-94 and A. terreus F-98 cellulases produced from rice straw throughout SSF, with activities (CMCase 161 IU/mL and FPase 76 IU/mL) and (CMCase 232 IU/mL and FPase 80 IU/mL), respectively; expressed good yield of TRS ≈ 19 g/L and 24 g/L with saccharification% of ≈ 50 and 61% , respectively.
Roslan et al. (2011) reported production of cellulase enzyme from Aspergillus sp., using rice straw as a substrate throughout a SSF process. The produced crude enzyme was used for enzymatic hydrolysis of milled and autoclaved rice straw without any acid or alkali pretreatment. It was found that enzymatic hydrolysis of milled and autoclaved rice straw produced higher concentration of glucose than that of milled rice straw, and suggested that thermal treatment on milled rice straw facilitated enzymatic hydrolysis due to creation of amorphous region in starch molecules when it was heated. Additionally, heating up also made the water in the medium partially acidic due to the release of acetyl group from hemicelluloses. This explained the increase in saccharification (Bacon et al., 1981).
Whole fungal cells hydrolysis and saccharification of rice straw
It was obvious from results illustrated in Figure.1, that addition of inorganic nutrients does not significantly increase the production of TRS. Hydrolysis and saccharification of ground and steam-pretreated (autoclaved) rice straw with T. viride F-94 produced TRS of ≈ 15 g/L and 16 g/L, with % saccharification of ≈ 39% and 41%. A. terreus F-94 produced TRS of ≈ 17 g/L and 17.3 g/L, with % saccharification of ≈ 44% and 45%, in absence and presence of nutrients, respectively.
Gomes et al. (2006) reported that; lignocellulosic degrading organisms have been used for the conversion of lignocellulosic materials into soluble sugars or solvents in several biotechnological and industrial applications. Fang et al. (2009) suggested solid state fermentation SSF of lignocellulosic wastes by whole fungal cells, since enzymes should be produced in situ and used in crude form to reduce the cost. According to Chinedu et al. (2010); fungi can produce extracellular cellulase enzymes that break down cellulose into two or three glucose units which can be readily degraded and assimilated as glucose monomers.
It can be estimated from data illustrated in Figure.1., that enzymatic saccharification of rice straw was better than that of whole fungal cell saccharification and recording higher yield of TRS.
Havannavar and Geeta (2007) reported that when microbiological and enzymatic pretreatments are compared, enzymatic pretreatment is more effective in obtaining the reducing sugars, which could be the source of useful products such as bioethanol production.
It can be concluded from all the above results obtained from this study that; A. terreus F-98 expressed higher enzymatic production and saccharification and better yield of TRS in hydrolysis and saccharification of rice straw using whole fungal cells than that of T. viride F-94. These observations can be correlated to the source of isolation of F-98 and F-94; from rice straw and bagasse, respectively (Abo-State et al., 2012). Thus A. terreus F-98 enzymes are well adapted to rice straw substrate.
In this study; although EHF produced higher yield of TRS sugars than that of FHS in SSF process of rice straw, would suggest the use of the cellulases enzymes produced through SSF by fungi for direct saccharification of agricultural lignocellulosic wastes with considerable efficiency. However the large amounts of enzymes (1:14 w/v substrate:cellulases) required for enzymatic conversion of hemicellulose and cellulose to fermentable sugars would impact severely on the cost effectiveness of this technology. So SSF by Fungi without addition of nutrients is recommended for further studies and processes as the overall cost and required power would be lower.
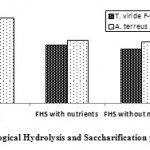 |
Figure 1: Different Biological Hydrolysis and Saccharification processes of Rice Straw.
|
Bioethanol fermentation
Evaluation of ethanol production is necessary to quantify the process final performance. In this study; the hydrolyzates resulted from hydrolysis and saccharification of rice straw in SSF by Trichoderma viride F-94 and Aspergillus terreus F-98 were used for ethanol fermentation by different yeast isolates throughout SHF processes and produced nearly the same ethanol concentrations. SHF of rice straw by T. viride F-94 and C. tropicalis Y-26 or S. cerevisiae Y-39 produced ethanol concentration of ≈ 13 g/L and 12 g/L with ethanol yield of ≈ 93.94% and 98.21%, respectively. SHF of rice straw by A. terreus F-98 and C. tropicalis Y-26 or S. cerevisiae Y-39 produced ethanol concentration of ≈ 11 g/L and 15 g/L with ethanol yield of ≈ 86.64% and 99.09%, respectively. The discrepancy between ethanol concentrations and ethanol yields might be attributed to the efficiency of each yeast isolate for utilization of different types of reducing sugars present in the hydrolyzates.
According to Chandel et al. (2011); ethanol production from different lignocellulosic sources depends upon many factors like the initial sugar concentration in the hydrolysate; strain used for fermentation; presence of the inhibitory compounds and the employed cultivation conditions.
As representative samples; determination of mono-saccharides in hydrolyzates of rice straw produced throughout SHF by T. viride F-94 and C. tropicalis Y-26 and A. terreus F-98 and S. cerevisiae Y-39 was done and illustrated in Figure.2. FHS of rice straw with T. viride F-94 afforded the following: glucose (6.99 g/L), xylose (3.10 g/L) Rhamnose (3.59 g/L) and arabinose (1.5 g/L). While that with A. terreus F-98 led to glucose (10.59 g/L), xylose ( 7.51 g/L), rhamnose ( 1.10 g/L) and arabinose (0.49 g/L). The recorded variations in the produced mono-saccharides yields can be attributed to different capabilities of each fungal isolate for delignification and hydrolysis of cellulose and hemi-cellulose in rice straw. FHS of rice straw in SSF with A. terreus F-98 led to higher delignification effect than that of T. viride F-94 recording ≈ 34 and 26 %, respectively. This delignification might explain the higher TRS yield obtained in F-98 hydrolysate (≈ 17 g/L) than that of F-94 (≈ 15 g/L) as cellulose and hemi-cellulose would be more available for saccharification. T. viride F-94 and A. terreus F-98 expressed higher degradation effect on hemi-cellulose (38% and 52%, respectively) than that of cellulose (28% and 41%, respectively).
Senthilgura et al. (2011) reported that cellulose hydrolysis yields glucose which can be readily fermented with many existing organisms. Hemi-cellulose hydrolysis produces both hexoses and pentoses (manose, galactose, xylose and arabinose) that are not all fermented with exiting strains.
According to Nigam (2001); efficient utilization of the hemicelluloses component of lignocellulosic feedstock (25% of dry weight of hardwood and predominantly D-xylose) offers an opportunity to reduce the cost of producing fuel ethanol by 25%. Jaáfaru and Fagade (2007) reported that, lignin physically encrusts cellulose that makes it resistant to enzymatic degradation. This leads to shortage of utilizable carbohydrates in lignin-rich substrates and consequently poor growth of organism and low enzyme yield. Jahromi et al. (2011) reported degradation of 32.86 % hemicelluloses and 16.3% cellulose in SSF of rice straw supplemented with 1% urea as a nitrogen source with 8 d of incubation at 25oC using A. terreus ATCC-74135 and recorded production of TRS 17.63 mg/g with total weight loss of 12.27%. Umasaravanan et al. (2011) reported lignocelluloses degradation in rice straw by A. tamari over a period of 21 d. The percentage degradation for cellulose and lignin was 39% and 3%, respectively. According to Salvachua et al. (2011); the selected fungal strain for fungal pretreatment of lignocellulosic wastes should be the one giving the highest amount of fermentable sugars in the shortest period of time.
In this work, the good delignification efficiency and utilization of hemi-cellulose and cellulose with good yield of TRS within relatively short incubation period (7d) add to the advantages of T. viride F-94 and A. terreus F-98.
It is obvious from Figure.2, that the two yeast isolates have good capabilities for utilization of pentoses and hexoses. Complete consumption of arabinose, glucose and rhamnose with lower consumption (≈ 77%) of xylose was recorded in SHF by T. viride F-94 and C. tropicalis Y-26. In case of SHF by A. terreus F-98 and S. cerevisiae Y-39, only complete consumption occurred for glucose and rahmnose, while arabinose and xylose recorded nearly the same consumption efficiency of ≈ 59% and 57%, respectively.
Similar observation was reported by Yadav et al. (2011), where batch fermentation of pretreated rice straw hydrolyzate with 30 g/L TRS produced ethanol of 7.5 g/L after 36 h incubation with a yield of 0.3 g/g and productivity of 0.2 g/L h. The ethanol efficiency was low i.e. 55%. The reason may be due to most of xylose was left unfermented by S. cerevisiae OVB11, as the hydrolyzate contains both glucose and xylose.
It has been reported by Girio et al. (2010) and Salvachua et al. (2011) that evaluation of ethanol production is necessary to quantify the process final performance. At industrial level, only glucose is being fermented with high ethanol production yields while xylose fermentation, which is also essential for the economical success of lignocellulosic ethanol, continues being investigated to raise the low yields obtained so far. Thus the ability of the two locally isolated Candida tropicalis Y-26 and Saccharomyces cerevisiae- Y-39 to ferment glucose and xylose in the obtained hydrolyzates to ethanol is great advantage.
Patel et al. (2007) reported that grinding and autoclaving of 10 g/L rice straw has resulted in release of TRS of 14 mg/g and with fungal treatment in presence of nutrients (Mandel’s medium), still slight increase in the release of sugars was observed due to the enzymatic hydrolysis of celluloses by the fungal enzymes. The yield of TRS after SmF of rice straw by Aspergillus awamori, A. niger, T. reesi and T. viride was ≈ 16, 16, 15 and 15 mg/ g, respectively. Ethanol yield after the whole SHF process of 12 d by S. cerevisiae amounted to 1.6, 1.8, 1.6 and 1.5 g/L, respectively.
Kumar and puspha (2012) reported fungal treatment of milled and autoclaved rice straw (5 g/100 mL Mandel’s medium) for 5 d of incubation at 30oC resulted in production of TRS yield of 73.7 mg/g and 62.7 mg/g in case of T. reesei and A. awamari, respectively. Ethanol yield after the whole SmF process of 12 d by Zymomonas mobilis strain amounted to ≈ 8.7, 4.1, 7.9 g/L, respectively.
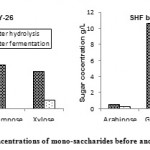 |
Figure 2: Concentrations of mono-saccharides before and after fermentation.
|
Conclusions
It can be confirmed from this study that cellulase enzymes produced from Egyptian rice straw using locally isolated fungal strains T. viride F-94 and A. terreus F-98 are highly suitable for use with pretreated ground and steam-pretreated (autoclaved) rice straw. However the large amounts of enzymes (1:14 w/v substrate:cellulases) required for enzymatic conversion of hemi-cellulose and cellulose to fermentable sugars would impact severely on the cost effectiveness of this technology.
Generally; worlwide, hydrolysis and sacchaification step is carried out by commercially available cellulase enzyme which is very expensive.This preliminary study revealed that ethanol production from ground and steam pretreated (autoclaved) rice straw is possible through separte hydrolysis and fermentation SHF process by intact fungal organisms as source of cellulase and hemi-cellulase enzymes for hydrolysis and saccharification in solid sate fermentation SSF and yeast for fermentation of produced sugars in the hydrolysates. A significant removal of lignin from rice straw was achieved through this eco-friendly process, which increased the availability of cellulose and hemi-cellulose, producing good yield of total reducing sugars TRS and consequently high production of ethanol.
The ethanol yields in this study were ≈ 50 and 39 gallons/ton in case of SHF processes with T. viride F-94 and C. tropicalis Y-26 or S. cerevisiae Y-39, respectively. While that, in case of SHF processes with A. terreus F-98 and C. tropicalis Y-26 or S. cerevisiae Y-39, recorded ≈ 38 and 45 gallons/ton, respictevly. These ethanol yields were respectively; 2.2, 2.82, 2.89 and 2.44 times lower than the theoretical yield of rice straw (109.9 gallons/ton) as per National Renewable Energy Laboratory (NREL) calculation, which is found at (http: // www1.eere. energy.gov/ biomass/ ethanol yield calculation.html).
Further study is needed to optimize the conditions for maximum production of TRS and ethanol from rice straw. The location of Egypt, large manpower and the availability of high energy lignocellulosic wastes suggest that cellulases enzymes and biofuel industries are highly feasible in Egypt if there is an access to low cost proper conversion and fermentation technologies.
References
- Abd El-Zaher, F.H. and Fadel, M. (2010) “Production of bioethanol Via Enzymatic Saccharification of Rice Straw by Cellulase Produced by Trichoderma Reesei under solid state fermentation”, New York Sci. J., 72-78.
- Abo-State, M.A., Ragab, A.M.E., EL-Gendy, N.Sh., Farahat L.A. and Madian, H.R. (2012) “Effect of different pretreatments on Egyptian sugar-cane bagasse saccharification and bioethanol production” Egyptian J. Petrol. (Accepted in Press).
- Abo-State, M.A.M. (2003) “Production of carboxymethyl cellulase by Fusarium oxysporium and Fusarium neoceros from gamma-pretreated lignocellulosic wastes”,vEgypt. J. Biotechnol., 15: 151-168.
- Abo-State, M.A.M., Hammad, A.I., Swelim, M. and Gannam, R.B. (2010) “Enhanced production of cellulase (s) by Aspergillus isolated from agriculture wastes by solid state fermentation”, American-Eurasian J. Agric. Environ. Sci., 8(4): 402-410.
- Abou Hussein, S.D. and Sawan, M. (2010) “The utilization of agricultural waste as one of the environmental issues in Egypt (A case study)” J. Appl. Sci. Res., 6(8): 1116-1124.
- Alvira, P., Tomas-Pejo, M., Ballesteros, M. and Negro, M.J. (2010) “Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review”. Bioresour. Technol., 101: 4851-4861.
- Askar, M.M.S., Ahmed, Y.M. and Ramadan, M.F. (2009) ‘Chemical characteristics and antioxidant activity of exopolysaccharides fractions from Microbacterium terregens” Carbohydrate polymers, 77: 563-567.
- Azzaz, H.H., Murad, H.A., Kholif, A.M., Hanfy, M.A. and Abdel Gawad, M.A. (2012) “Optimization of culture conditions affecting fungal cellulase production” Res. J. Microbiol. 7(1): 23-31.
- Bacon, J.S.D., Chesson, A. and Gordon, A.H. (1981) “Deacetylation and enhancement of digestibility”, Agr. Environ., 6:115-126.
- Chandel, A.K., Chandrasekhar, G., Radhika, K., Ravinder, R. and Ravindra, P (2011) ” Bioconversion of pentose sugars into ethanol: A review and future directions”, Biotechnol. Mol. Biol. Rev., 6(1): 8-2.
- Chandra, M.S., Viswanath, B. and Reddy, B.R. (2007) “Cellulolytic enzymes on lignocellulosic substrates in solid state fermentation by Asperigillus niger”, Indian J. Microbiol., 47: 323-328.
- Chinedu, S.N., Eni, A.O., Adeniyi, A.I. and Ayangbemi, J.A. (2010) “Assessment of growth and cellulase production of wild-type microfungi isolated from Ota, Nigeria”, Asian J. Plant Sci., 9 (3): 118-125.
- Dogaris, I., Vakontios, G., Kalogeris, E., Mamma, D. and Kekos, D. (2006) “Induction of cellulases and hemicellulases from Neurospora crassa under solid-state cultivation for bioconversion of sorghum bagasse ethanol”, Ind. Crops and Products, 29: 404-411.
- El-Dorghamy, A. (2007) “Energy and Environmental Management in Egypt”, MSc. Thesis, Royal Institute of Technology, Stockholm.
- Fang, X., Yano, S., Inoue, H. and Sawayama, S. (2009) “Strain improvement of Acremonium cellulolyticus for cellulase production by mutation” J. Biosci. Bioeng., 107:256-261.
- Fawzi, E.M. and Hamdy, H.S (2011) “Improvement of carpoxy methyl cellulose production from Chaetomium cellulolyticum NRRL 18756 by mutation and optimization of solid state fermentation”, Bangladesh J. Bot., 40(2): 139-147.
- Gao, J., Weng, H., Zhu, D., Yuan, M., Guan, F. and Xi, Y. (2008) “Production and characterization of cellulolytic enzymes from thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover”, Bioresour. Technol., 99: 7623-7629.
- Girio, F.M., Fonseca, C., Carvalheiro, F., Duarte, L.C., Marques, S., Bogel-Lucasik, R. (2010) “Hemicelluloses for fuel ethanol: A review”, Technol., 101: 4775-4800.
- Gomes, I., Shaheen, M., Rahman, S.R. and Gomes, J. (2006) “Comparative studies on production of cell wall degrading hydrolases by Trichoderma reesei and T. viride in submerged and solid state cultivations”, Bangladesh J. Microbiol., 23(2):149- 155.
- Gunasekaran, P. and Kamini, N.R. (1991) “High ethanol productivity from lactose by Immobilized cells of Kluyveromyces fragilis and Zymomonas mobilis“, World J. Microbiol. Biotechnol., 7: 551-556.
- Harmsen, P., Huijgen, W., Bermudez, L., Bakker, R. (2010), “Literature review of physical and chemical pretreatment processes for ligno-cellulosic biomass” BIOSYNENERGY, Report 1184, http://www.biomassandbioenergy.nl/ricestraw.htm
- Havannavar, R.B. and Geeta, G.S. (2007) “Bioethanol production from enzyme hydrolysed agroresidues”, Karnataka J. Agric. Sci., 20(4): 871-872.
- Helal, A.G. (2006) “Bioconversion of straw into improved fodder: preliminary treatment of rice straw using mechanical, chemical and /or Gamma irradiation” Microbiology, 34 (1): 14-21.
- Irfan, M., Gulsher, M., Abbas, S., Sayed, Q., Nadeem, M. and Baig, S. (2011) ” Effect of various pretreatment conditions on enzymatic saccharification”, Songklanakarin J. Sci. Technol., 33 (4), 397-404.
- Jaáfaru, M.I. and Fagade, O.E. (2007) “Cellulase production and enzymatic hydrolysis of some selected local lignocellulosic substrates by a strain of Aspergillus niger. Res. J. Biol. Sci., 2 (1): 13-16.
- Jahromi, M.F., Liang, J.B., Rosfarizan,, Goh, Y.M., Shokryazdan, P. and Ho, Y.W. (2011) “Efficiency of rice straw lignocelluloses degradability by Aspergillus terreus ATCC 74135 in solid state fermentation” African J.Biotechnol.,10 (21): 4428-4435.
- Kang, S.W., Park, Y.S., Lee, J.S., Hong, S.I. and Kim, S.W. (2004) “Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass”, Bioresour. Technol., 91 (2): 153-156.
- Kocher, G.S., Kalra, K.L. and Banta, G. (2008) “Optimization of cellulase production by submerged fermentation of rice straw by Trichoderma harzianum Rut-C 8230″ The Int. J. Microbiol, 5(2): DOI: 10.5580/1658.
- Kumar, S.A. and Pushpa, A. (2012) “Saccharification by fungi and ethanol production by bacteria using lignocellulosic materials”, Int. Res. J. Pharmacy, 3(5): 411-414.
- Miller, G.L. (1959) “Use of dinitrosalysilic acid reagent for the determination of reducing sugars”, Anal. Chem., 31: 426-428.
- Nigam, N.J. (2001) “Ethanol production from wheat straw hemicelluloses hydrolysate by Pichia stipitis“, J. Biotechnol., 87: 17-27.
- Oxoid (1982): Manual of Culture Media, Ingredients and Other Laboratory Services. Publised by Oxoid Limited, Wade Road, Basingstoke, Hampshire RG24OPW. UK (1982).
- Pandey, A., Nigam, P., Soccol, C.R., Soccol, V.T., Singh, D. and Mohan, R. (2000) “Advances in microbial amylases”, Biotechnol. Appl. Biochem., 31: 135–152.
- Patel, S.J., Onkarappa, R. and Shobha K.S. (2007) ” Comparative study of ethanol production from microbial pretreated agricultural”, J. Appl. Sci. Environ. Manage., 11(4) 137 – 141.
- Raimbault, M. (1998) “General and microbiological aspects of solid substrate fermentation”, Electronic J. Biotechnol. ISSN: 0717-3458.
- Roslan, A.M., Yee, P.L., Shah, U.K.M., Aziz, S.A. and Hassan, M.A. (2011) “Production of bioethanol from rice straw using cellulase by local Asperigllus” Int. J. Agric. Res., 6: 188-193.
- Salvachua, D., Prieto, A Lopez-Abelairas, M., Lu-Chau, T.,Martinaz, T. and Martinez, M.J. (2011) “Fungal pretreatment: An alternative in second-generation ethanol from wheat straw” Bioresour. Technol., 102: 7500-7506.
- Senthilguru, K., George, T.S., Vasanthi, N.S. and Kannan, K.P. (2011) “Ethanol production from lignocellulosic waste” World J. Sci.Technol. 1(11): 12-16.
- Sher, M.G., Abbas, S., Irfan, M. and Nadeem, (2010) “Biosynthesis of cellulase from bagasse with fungal strains”, Pak. J. Biochem. Mol. Biol., 43(3):146-148.
- Singhania, R.R., Patel, A.K., Soccol, C.R. and Pandey, A. (2009) “Recent advances in solid-state fermentation”, Biochem. Eng. J., 44: 13-18.
- Sukumaran, R.K., Surender, V.J., Sindhu, R., Binod, P., Janu, K.U., Sajna, K.V., Ajasree, K.P. and Pandey, A.V. (2010) “Lignocellulosic ethanol in India: Prospects, challenges and feedstock availability”, Bioresour. Technol., 101: 4826-4833.
- Uma, C., Muthulakshmi, C., Gomathi, D. and Gopalakrishnan V.K. (2010) “Fungal invertase as aid for production of ethanol from sugarcane bagasse”, Res. J. Microbiol. 5(10): 980-985.
- Umasaravanan, D., Jayapriya, J. and Rajendran R. (2011) “Comparison of lignocelluloses biodegradation in solid state fermentation of sugarcane bagasse and rice straw by Aspergillus tamari“, Cey. J. Sci. (Bio. Sci.) 40 (1): 65-68.
- Umikalsom, M.S., Arrif, A.B., Shamsuddin, Z.H., Tong, C.C., Hassan, M.A. and Karim, M.I.A. (1997) “Production of cellulase by a wild strain of Chaetomium globosum using delignified oil palm empty-fruit-bunch fiber as substrate”, Appl. Microbiol. Biotechnol. 47: 590-595.
- Wickerham, L. J. (1951): Taxonomy of yeasts. US. Dept. Technical bulletin No.1029:1-56.
- Yadav, K.S., Naseeruddin, S., Prashanthi, G.S., Sateesh, L. and Rao, L.V. (2011) “Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis“, Technol. 102(11):6473-6478.
- Yang, Y.H., Wang, B.C., Wang, Q.H., Xiang, L.J. and Duan, C.R. (2004) “Research on solid-state fermentation on rice chaff with a microbial consortium”. Colloid Surf., 34: 1-6.16.
- Yu, Z. and Zhang, H. (2004) “Ethanol fermentation of acid – hydrolyzed cellulosic pyrolysate with Saccharomyces cerevisiae“, Bioresour. Technol., 93: 199-204.

This work is licensed under a Creative Commons Attribution 4.0 International License.





