How to Cite | Publication History | PlumX Article Matrix
A. Amatussalam1, R. Babu Rajendran2 and M. N. Abubacker3*
1Department of Chemistry, National College, Tiruchirappalli-620 001, India.
2Department of Environmental Biotechnology, Bharathidasan University, Tiruchirappalli-620 024, India.
3Department of Botany, National College, Tiruchirappalli-620 001, India.
Corresponding Author E-mail: abubacker_nct@yahoo.com
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1061
ABSTRACT:
Heavy metal contamination due to natural and or anthropogenic sources is a global environmental concern. Removal of heavy metal copper was carried out using the fungus Fusarium oxysporum (NCBT-156) strain in copper sulfate solution with 10 percent Czapek-dox liquid medium. Biosorbent matrix was developed using Carica papaya dried stem to colonize the fungal strain. Fungal mycelium acts as binding sites for copper accumulation at the hyphal tips. Maximum efficiency of copper removal by biosorption upto 90 percent was achieved at the end of 5th day of incubation for 100 and 200 ppm, upto 80 percent for 300 and 400 ppm, and upto 70 percent for 500 ppm concentrations. SDS-PAGE protein profile showed significant difference in 66 KDa protein band after copper absorption by the fungus. FTIR spectroscopic analysis revealed that the main functional groups involved in the uptake of copper by F. oxysporum were carbonyl, carboxyl, amino and hydroxyl groups.
KEYWORDS: Bioabsorption; Bioaccumulation; Bioremediation; Biosorption; copper; Fusarium oxysporum.
| Copy the following to cite this article: Amatussalam A, Rajendran R. B, Abubacker M. N. In situ Carica papaya Stem Matrix and Fusarium oxysporum (Ncbt-156) Mediated Bioremediation of Copper. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Amatussalam A, Rajendran R. B, Abubacker M. N. In situ Carica papaya Stem Matrix and Fusarium oxysporum (Ncbt-156) Mediated Bioremediation of Copper. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10096 |
Introduction
Heavy metals are conventionally defined as elements with metallic properties like conductivity, stability as cations, ligand specificity, and atomic number > 20. The most common heavy metal contaminants are Cd, Cr, Cu, Hg, Ni and Pb. Heavy metals are known to be toxic to plants and in most organisms when present in soils in excessive concentrations (Jing Yan-de et al., 2007).
However contamination of heavy metals in the environment is a major global concern because of their toxicity and threat to human life and environment (Ceribasi and Yetis, 2001; Hernandez et al., 1998). A sudden boost in the industrial activities has contributed quantitatively as well as qualitatively to the alarming increase in the discharge of metal pollutants into the environmental sink, especially the aqueous environment. Dispersion of the metal ions in water bodies leads to their bioaccumulation and biomagnification through the food chain and results in increased toxicity (Ranigupta and Mohapatra, 2003). Disposal of industrial and urban wastes into the soil and water bodies violating stringent regulation in many countries including India has led to disastrous consequences to the ecosystems. Due to excessive loading of these wastes beyond their self-cleaning capacities, the ecosystems have resulted in decreased availability of clean potable water and normal soil for crop production. Compared to the organic wastes, inorganic wastes like heavy metals, pose a great threat, as they cannot be completely removed or degraded from the ecosystem (Kamaludeen et al., 2003).
Copper contamination of the environment is largely due to its release by industrial units producing non-ferrous metals, fertilizers, disposal of tailings or solid wastes from mines and from fly-ash produced by combustion of coal and organic matter. Copper wastes are discharged into the environment as a consequence of industrial and manufacturing activities (Gadd and White, 1993). Considerable attention has been focused on bio-remediation of heavy metals using bacteria and fungi in recent years due to public and scientific awareness regarding the release of such pollutants from the industries (Kartal et al., 2004). Biosorption or bioaccumulation of metals from aqueous solutions using varying species of fungi such as Aspergillus niger (Magyarosy et al., 2002; Dursun et al., 2003), Rhizopus nigricans (Bai and Abrahanh, 2002; Dursun et al., 2003), Mucor rouxii (Yan and Viraraghavan, 2003) and Trichoderma atroviride (Errasquin and Vazquez, 2003) have been studied.
The current paper discusses a study that evaluated the ability of Fusarium oxysporum NCBT-156 strain in bioremediation of copper element in copper sulfate solution in situ condition.
Materials and Methods
Stock solutions of copper (10 g/l) was prepared by dissolution of copper sulfate (CuSO4) in deionized water. From the stock solution various concentrations of test samples of 100 ppm to 1000 ppm were prepared.
Fungal culture
The fungus used for the study was Fusarium oxysporum NCBT-156 strain isolated from the tannery effluent affected soils of Sempattu, Tiruchirappalli. The strain was deposited in the culture collection centre at the Microbiology Laboratory, Department of Botany, National College, Tiruchirappalli in polyurethane foam immobilization and stored for further experimental use.
Preparation of inoculum
From the immobilized culture, the fungus F. oxysporum (NCBT-156) was inoculated in Czapek-Dox liquid medium (Booth, 1971) for 48 hours for the establishment in the culture medium. Then the test samples of copper sulfate solution were added individually for each concentration in a conical flask with 10% Czapek-Dox liquid medium (90 ml copper sulfate solution + 10 ml Czapek-Dox liquid medium) and the cultures were incubated at 28°C ± 1°C and observed for biosorption of metal copper.
Biosorption matrix
Dried stem material of Carica papaya was prepared as biosorbent matrix for Fusarium oxysporum (NCBT-156) to reduce the copper with different concentration. This is important in the present day scenario, as there is an increasing need for an effective and economical process to remove metal ions from the industrial wastewater. A one-year old plant stem was cut into small pieces, dried in shade, sterilized and then used as biosorption matrix (Fig. 3a). The fungal strain was inoculated on the matrix and allowed for 48 hours for its establishment. The matrix along with fungal strain was then introduced into the copper sulfate solution + 10 ml Czapek-Dox liquid medium to facilitate bioremediation process (Fig. 3d).
Colorimetric analysis
Colorimetric analysis was employed for monitoring copper decolourization by the fungal strain. Prior to this, the absorbance maxima of copper incorporated broth was determined. The absorption maxima was at 380 nm and hence decolourization was assayed at this absorption maxima. In order to avoid the interference of fungal mycelia in the absorbance value, the culture broth was centrifuged at 4000 rpm for5 minutes and the supernatant was analysed colorimetrically (380 nm). Aliquots were withdrawn from the culture broth at 24 hr interval for a period of 10 days and the extent of decolourization was monitored colorimetrically.
Decolourization assay
Decolourization was calculated in terms of percentage, using the following formula as described by Sani and Banerjee (1999).
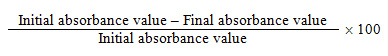
The fungal isolates were tested for their capacity to utilize the copper as mineral source. Two sets of mineral salt medium (Walker et al., 1993) of 100 ml capacity were prepared. To one set, 1% glucose as carbon source and copper at ten different concentrations of 100 to 1000 ppm as mineral source were added. To another set ammonium nitrate (0.03 gm/100 ml) as nitrogen source and the copper was incorporated at ten different concentrations of 100 to 1000 ppm as mineral source were added. Both sets were inoculated with Fusarium oxysporum NCBT 156 culture and incubated at 28° ± 1° C for 10 days. At the regular interval of the inoculation periods, the culture broth was assayed colorimetrically for copper decolourization.
SDS-PAGE protein profile
SDS-polyacrylamide Gel Electrophoresis (SDS-PAGE) protein profile (Laemmli, 1970) was performed for F. oxysporum NCBT-156 before and after the copper absorption.
Fourier Transform Infrared (FTIR) spectroscopy analysis
FTIR spectroscopy was used to detect the vibration frequency changes in F. oxysporum (NCBT 156) strain biomass before and after the copper uptake. The spectra were collected by Perkin Elmer spectrometer with the range 4000-400 cm–1 using chloroform as the mulling agent. The background obtained from the scan of pure chloroform was automatically subtracted from the sample spectra.
Results and Discussion
Biosorption of copper
Biosorption of copper by Fusarium oxysporum NCBT-156 strain manifested by decolourisation of copper sulphate solution is presented in Table-1. In both normal and matrix mediated processes biosorption is effective upto 500 ppm and beyond which a steady status is maintained upto 1000 ppm under experimental conditions. Bioaccumulation of copper had occurred in the mycelial matrix as well as in the hyphal tips which is evident. As a result, F. oxysporum NCBT-156 exhibited non-sporulation condition whereas the control produced micro or macro conidia (Fig. 1a-f, 2a-e, 3b and d).
Table 1: Copper decolourization by F. oxysporum NCBT-156.
| Copper
conc. ppm |
Percentage of decolourization / Days | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 100 | 50 ± 0.8164 | 70 ± 0.9914 | 80 ± 0.8164 | 85 ± 0.9430 | 90 ± 0.4778 | 90 ± 0.8164 | 90 ± 0.8164 | 90 ± 0.4778 | 90 ± 0.8164 | 90 ± 0.4778 |
| 200 | 45 ± 0.4724 | 70 ± 0.9914 | 80 ± 0.8164 | 85 ± 0.9430 | 90 ± 0.4778 | 90 ± 0.4778 | 90 ± 0.8164 | 90 ± 0.8164 | 90 ± 0.8164 | 90 ± 0.8164 |
| 300 | 45 ± 0.4724 | 70 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.9430 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.9430 |
| 400 | 40 ± 0.4743 | 60 ± 0.9427 | 70 ± 0.9914 | 70 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.8164 | 80 ± 0.9430 | 80 ± 0.9430 |
| 500 | 40 ± 0.8124 | 60 ± 0.9427 | 70 ± 0.8164 | 70 ± 0.8164 | 70 ± 0.9914 | 70 ± 0.9430 | 70 ± 0.9914 | 70 ± 0.8164 | 70 ± 0.8164 | 70 ± 0.4778 |
| 600 | 40 ± 0.4743 | 60 ± 0.9427 | 60 ± 0.9427 | 70 ± 0.9914 | 70 ± 0.9914 | 70 ± 0.4778 | 70 ± 0.4778 | 70 ± 0.8164 | 70 ± 0.9430 | 70 ± 0.8164 |
| 700 | 35 ± 0.4739 | 50 ± 0.8164 | 60 ± 0.9427 | 70 ± 0.8164 | 70 ± 0.8164 | 70 ± 0.4778 | 70 ± 0.8164 | 70 ± 0.9430 | 70 ± 0.8164 | 70 ± 0.4778 |
| 800 | 35 ± 0.4739 | 50 ± 0.8164 | 60 ± 0.9427 | 70 ± 0.9914 | 70 ± 0.8164 | 70 ± 0.8164 | 70 ± 0.9914 | 70 ± 0.4778 | 70 ± 0.9914 | 70 ± 0.9430 |
| 900 | 30 ± 0.4724 | 50 ± 0.8164 | 60 ± 0.9427 | 70 ± 0.9914 | 70 ± 0.8164 | 70 ± 0.9914 | 70 ± 0.4778 | 70 ± 0.9914 | 70 ± 0.9914 | 70 ± 0.9914 |
| 1000 | 30 ± 0.8164 | 50 ± 0.8164 | 60 ± 0.9427 | 70 ± 0.8164 | 70 ± 0.9914 | 70 ± 0.9430 | 70 ± 0.9914 | 70 ± 0.8164 | 70 ± 0.4778 | 70 ± 0.8164 |
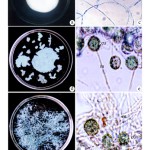 |
Figure 1
|
Percentage of decolourization
The percentage of decolourization of copper was maximum (90%) at the end of 5th day of incubation in 100 and 200 ppm concentrations, 80% in 300 and 400 ppm concentrations and 70% in 500 to 1000 ppm concentrations (Fig. 2a-f).
 |
Figure 2
|
According to Nealson et al. (1992), the metal removal from a solution is achieved through enzymatic system of fungus. Garbisn and Alkorta (2001) reported that heavy metals cannot be destroyed biologically but are only transformed from one oxidation state to another. Such transformation mechanisms might have occurred in copper decolourization by F. oxysporum strain.
Metal remediation strategies using microorganisms can minimize the bioavailability and biotoxicity of heavy metals as reported by Gadd (2000). Microbial approach for metal detoxification affords the potential for selective removal of toxic metals, operation flexibility and easy adaptability for in situ and ex situ application (Lloyd and Lovely, 2001). The Fusarium oxysporum NCBT-156 in Carica papaya stem matrix (Fig. 3a,c and d) used in this study can serve as a bioremediating agent in the application in a range of bioreactor configurations to reduce the pollutants.
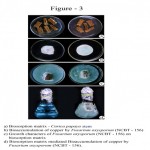 |
Figure 3
|
Biosorption mechanism
The mechanisms by which metal ions bind to the cell surface include electrostatic interactions, Van der Waals forces covalent bonding, redox interactions and extracellular precipitation or combination of these processes (Blanco, 2000). The negatively charged groups (carboxyl, hydroxyl and phosphoryl) of the fungal cell wall absorb metal cations, which are then retained by mineral nucleation (Blanco, 2000). The microbial metal resistance as well as remediation might be due to the presence of plasmids encoded specific metal conferring resistance to a variety of metals including copper as reported by Mergeay (1991). The biosorption occurred in F. oxysporum NCBT-156 in copper medium might have similar mechanisms for their resistance and the bioaccumulation phenomenon as reported by Mergeay (1991) and Blanco (2000).
Non-sporulation mechanism
The non-sporulation of F. oxysporum NCBT-156 mycelium may be due to heavy metal stress using different defense systems such as exclusion, compartmentalization, formation of complexes and synthesis of binding proteins like metallothioneins (MTs) and phytochelatins (PCs). The toxicity mechanism of metal ions could be (i) blocking the essential biological functional groups of biomolecules especially proteins and enzymes, (ii) displacing the essential metal ion in biomolecules, and (iii) modifying the active conformation of biomolecules resulting in the loss of specific activity (Ochiai, 1997). Such toxicity mechanisms might have resulted in F. oxysporum NCBT-156 being unable to produce the micro or macro-conidia under copper stress environment in the experimental conditions.
SDS-PAGE protein profile
SDS-PAGE analysis replicated thrice (L1, L2 and L3) showing similar results for F. oxysporum (NCBT-156) before and after copper absorption. The protein profile showed a significant difference in 66 KDa protein band after copper absorption (Fig. 4a and b) and seems to be a genetically improved strain (Mitra et al., 2001).
 |
Figure 4
|
FTIR functional groups analysis
The functional groups involved in the absorption of copper by F. oxysporum (NCBT-156) biomss were determined using FTIR spectroscopic analysis. The main functional groups involved in absorption process were found to be carbonyl, carboxyl, amino and hydroxyl groups (Fig. 5). The involvement of these functional groups in the metal absorption process can be judged from the change in frequency of the absorbing groups (Fig. 5a and b). The absorbance of the peaks in copper absorbed mycelium and control (unabsorbed) showed changes in the peaks (Hanif et al., 2007).
 |
Figure 5
|
Summary and Conclusions
Biosorption of copper by F. oxysporum (NCBT-156) strains is an economically feasible and technically efficient technology for convenient metal ion removal, and can comfortably fit into the metal treatment processes. Further it is an eco-friendly approach as no further waste is generated into the environment. The industries generating metal polluted wastewater are to follow genetically improved F. oxysporum NCBT-156, Carica papaya stem biosorbent matrix as clean up technologies before discharging their liquid effluents into the water bodies to create healthy environment is recommended.
Acknowledgements
The authors AA and MNA thank DST-FIST, Government of India, New Delhi for providing the infrastructure facilities for the Departments of Chemistry and Botany, National College, Tiruchirappalli. The authors also thank Shri. K. Ragunathan, Secretary and Dr. K. Anbarasu, Principal, National College for their encouragement.
References
- Bai, R. S. and Abraham, T. L., Studies on enhancement of Ci(VI) biosorption by chemically modified biomass of Rhizopus nigricans. Water Res., 36: 1224-1236 (2002).
- Barnhart, T., Chromium chemistry and implication for environmental fate and toxicity. J. Soil., Contam. 6: 561 (1997).
- Blanco, A., Immobilization of non-viable cyanobacteria and their use for heavy metal adsorption from water in environmental biotechnology and cleaner bioprocesses. (ed. Oluguin, E. J., Sanchez and Hernandez, E.). Philadelphia Taylor and Amp. Francis, p. 135 (2000).
- Booth, C., Fungal culture media. In: Methods in Microbiology. Vol. 4. (ed. Norris, J. R. and Ribbous, D. W.), Academic Press, London, p. 66 (1971).
- Ceribasi, I. H. and Yetis, U., Biosorption of Ni(ii) and Pb(ii) by Phanaerochaete chrysosporium from a binary metal system kinetics. Water SA, 241: 15 (2001).
- Dursun, A. Y., Uslum, G., Tepe, O., Cuci, Y. and Ekiz, H. I., A comparative investigation on the bioaccumulation of heavy metal ions by urowini; Rhizopus arrhizus and Aspergillus niger. Biochem. Eng. J., 15: 87-92 (2003).
- Errasquin, E. L. and Vazquez, C. 2003. Tolerance and uptake of heavy metals by Trichoderma atroviride isolated from sludge. Chemosphere, 50: 137-143.
- Hanif, M. A., Nadeem, R., Bhatti, H. N., Ahmad, N. R. and Ansari, T. M., Ni (II) biosportion by Cassia fistula (Golden shower) biomass. J. Hazard Mater. B., 139: 345-355 (2007).
- Gadd, G. M., Bioremedial potential of metal mobilization and immobilization. Curr. Opi. Biotechnol., 11: 271 (2000).
- Gadd, G. M. and White, C., Microbial treatment of metal pollution – A working biotechnology. Trends Biotechnol., 11: 353 (1993).
- Garbisn, C. and Alkorta, I., Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresource Technol., 73: 229-236 (2001).
- Hernandez, A., Mellado, R. P. and Martinez, J. L., Metal accumulation and vanadium – induced multidrug resistance by environmental isolates of Escherichia herdmanni and Enterobacter cloaceae. Appl. Environ. Microbiol., 64: 4317 (1998).
- Jing Yan-de, Hezhen-li and Yang Xiao-e., Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. Sci. B., 8: 192-207 (2007).
- Kamaludeen, S. P. B., Arunkumar, K. R., Arudainayagam, S. and Ramasamy, R., Bioremediation of chromium contaminated environments. Indian J. Exp. Biol., 41: 972-985 (2003).
- Kartal, S. N., Munir, E., Kakitani, T. and Imamura, Y., Bioremediation of CCA-treated wood by brown-rot fungi Fomitopsis palusiris, Coniophora puteana and Laetiporus sulphureus. J. Wood Sci., 50: 182-188 (2004).
- Laemmli, U. K., Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685 (1970).
- Lloyd, J. R. and Lovely, D. R., Microbial detoxification of metals and radionuclides. Curr. Opi. Biotech., 12: 253 (2001).
- Magyarosy, A., Laidlaw, R. D., Kilaas, R., Echer, C., Clark, O. S. and Keasling, J. D., Nickel accumulation and nickel precipitation by Aspergillus niger. Appl. Microbiol. Biotech., 59: 382-388 (2002).
- Mergeay, M., Towards an understanding of the genetics of bacterial metal resistance. Tibtech., 9: 24 (1991).
- Mitra, J., Mukherjee, P. K., Kale, S. P. and Murthy, N. B. K., Bioremediation of DDT in soil by genetically improved strains of soil fungus Fusarium solani. Biodegradation, 12: 235-245 (2001).
- Nealson, K. H., Rosson, R. A. and Mayers, C. R., Microbial reduction of manganese and iron: New approaches to carbon cycling. App. Environ. Microbiol., 58: 439 (1992).
- Ochini, E., Bioinorganic Chemistry: An Introduction. Boston: Align and Bacon. p. 1 (1997).
- Rani Gupta and Mohapatr, H., Microbial biomass: An economical alternative for removal of heavy metals from waste water. Indian J. Exp. Biol., 41: 945-966 (2003).
- Sani, R. K. and Banerjee, U. C., Screening for organisms applicable to the decolourization of triphenylaniline dyes and optimization of biotransformation conditions in stirred tank reactor. Indian J. Environ. Ecoplan., 2: 1-9 (1999).
- Walker, A., Parekh, W. R., Roberts, S. J. and Welch, R., Evidence for the enhanced biodegradation of napropamide in soil. Pestic. Sci., 39: 55-60 (1993).
- Yan, G. and Viraraghavan, T., Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res., 37: 4486-4496 (2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.





