How to Cite | Publication History | PlumX Article Matrix
Morphogenesis in Catharanthus roseus G. Don
U. K. Sinha1 and Rachna Prasad2
1Department of Botany, University Professor and Dean, Faculty of Science, Patna University, Patna, India.
2Department of Botany, Magadh Mahila College, P.U., India.
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1084
ABSTRACT:
For in vitro propagation in Catharanthus roseus, the plant growth regulators BAP (cytokinin) and NAA (Auxin) were experimented in association with MS media. The amalgamation of BAP (0.5 mg/l) and NAA (1.0 mg/l) on MS basal evinced amplified exalted synergism with 100% cultured explants response and consequent morphogenesis.
KEYWORDS: 6-Benzyladenine (BAP); Catharanthus roseus; MS medium; Naphthalene acetic acid (NAA).
| Copy the following to cite this article: Sinha U. K, Prasad R. Morphogenesis in Catharanthus roseus G. Don. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Sinha U. K, Prasad R. Morphogenesis in Catharanthus roseus G. Don. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10239 |
Introduction
Catharanthus roseus is an important medicinal plant. It harbours a number of alkaloids in its different parts. Some of them are used as drugs to cure diseases like low blood sugar level, haemostatics (arrest bleeding), cancer, nervous disorder etc. This has aroused interest in the experimentation of the plant Its in vitro propagation has for reaching effect on enhanced alkaloids yield (Vanisree et al.. , 2004) and hence, quality medicines.
Material and Methods
In the present study, nodal explants (1-2 cm) were taken from healthy mother plants grown locally in Patna. Selection of suitable explants depend on various factors such as physiological stage, type of organ tissue, seasonal variation of parent plant from which the explants is selected (Muroshige, 1977). The explants were washed under running tap water for 10 minutes and then rinsed in 1% solution of cetanelon (1 l), a detergent. It was further washed in running tap water followed by sterile double distilled water. The explants were inoculated on incubated culture tubes with MS media supplemented with BAP (Cytokinin) and NAA (Auxin) in different concentrations. The media was prepared according to the standard procedure described (Gamborg and Werrter, 1975). The pH of the media was adjusted to 5.8 prior to sterilization done at 15 lles for 15 minutes and 121ºC.
The data of the experiments were recorded and analysed.
Results and Discussion
The nodal explants cultured on Murashige and skoog medium showed no response. However, the MS media supplemented with the plant growth regulators BAP and NAA in different gradations gave results. The best result amongst the other trials was endowed at 0.5 mg/l BAP in association with 1.0 mg/l NAA. At this amalgamation, all the cultured explants responded favourably i.e. 10% response was procured. It was followed by 0.5 mg/l BAP + 1.5 mg/l NAA with 66.6 response percentage and 3.3 cm shoot length. The concentration of 1.5 mg/l and 2.0 mg/l NAA respectively along with 0.5 mg/l BAP gave cultured plant response in unison at 50.83%. But, the shoot lengths differed at 5.2 cm and 4.3 cm respectively. The percentage of cultured explants responding devindled to 25% with a low of 2.2 cm. However, the conglomerate of BAP at 0.5 mg/l and NAA at 4.0 mg/l and beyond failed to stimulate any noticaeable morphogenesis.
Table 1: Effect of BAP (Cytokinin) + NAA (Auxin) + MS media on morphogenesis in Catharanthus roseus
| Sl. No. | Growth Regulator Conc. (mg/l (BAP+NAA) | No. of explants cultured | No. of explants responding | Response percentage | Shoot length in cm) |
| (i) | MS | 12 | Nil | Nil | Nil |
| (ii) | 0.5 + 0.5 | 12 | 4 | 33.33 | 4.1 |
| (iii) | 0.5 + 1.0 | 12 | 12 | 100.00 | 6.0 |
| (iv) | 0.5 + 1.5 | 12 | 8 | 66.66 | 3.3 |
| (v) | 0.5 + 2.0 | 12 | 7 | 50.83 | 5.2 |
| (vi) | 0.5 + 2.5 | 12 | 7 | 50.83 | 4.3 |
| (vii) | 0.5 + 3.0 | 12 | 3 | 25.00 | 2.2 |
| (viii) | 0.5 + 4.0 | 12 | Nil | Nil | Nil |
Effect of BAP+NAA with MS medium on Explant Response Percentage in morphogenesis in Catharanthus rose
 |
Graph 1
|
Effect of BAP+NAA with MS medium on Shoot length in morphogenesis in Catharanthus roseus
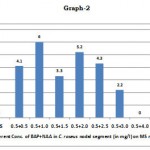 |
Graph 2:
|
It is well known that the balance of cytokinin and Auxin in plant tissues controls the direction of organogenesis (Skoog and Miller, 1975). Therefore, exogenous eytokinin and auxin are applied to keep this ratio favourable Here, this has been reaffirmed that there exists a synergism between BAP (Cytokinin) and NAA (Auxin) as depicted by the cultured explants responses. An efficient ins vitro regeneration system on medium enriched with BAP and NAA has been emphasised (Junaid et al., 2007). The responses were affirmative in the ratio of the PGRs BAP NAA till 1:6. The impetus was amplified at 0.5 mg/l BAP and 1.0 mg/l NAA with cent percent explants being responsive. But, when the concentration of NAA intensified beyond 4.0 mg/l with 0.5 mg/l of BAP, failure of in vitro propagation on MS media was witnessed. Gresshaff and Mohapatra (1981) reported that the morphogenic response is a function of chance since numerous paramenters are involved in the in vitro tissue experimentation based on trial and error method.
The hormonal balance within the tissue mass at specific sites (Thrope, 1978) and the physiological capacities of the tissue used (Jelaska, 1974) are noticed to determine the intrinsic control mechanism leading to morphogenesis.
Successful plant regeneration will be helpful in large scale multiplication of desirable genotype and also in inducing sufficient somaclonal variability for subsequent selection of desirable variants.
Acknowledgements
The second author (Rachna Prasad) greatfully acknowledge Prof. (Dr. U. K. Sinha for his able guidance during preparation of the manuscript.
References
- Vanisree M., Yue Lee C., Fung Lo S., Manohar Nalawade S., Yih Lin C., Sheng Tsay H., 2004. Studies on the production of some important secondary metabolities, Bot. Bull. Acad. Sin. 45 : 1-22.
- Murashige T., 1977 Clonal crops through tissue culture. In : Barz W., Reinhard E. and Zenk M.H. (Eds.). Proc. Life Sciences : Plant Tissue Culture and its Biotechnological Applications. Springer – Verlag, Berlin, pp : 392-403.
- Gamborg O.L. and Werrter L.R., 1975. Plant Tissue Culture Methods, NCR, Canada.
- Skoog F. and Miller C.O., 1957. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. soc. Exp. Biol. 11 : 118 – 130.
- Junaid A., Mujile A., Sharma M. and Tang W., 2007. Growth regulators affect primary and secondary somatic embryogenesis in Madagaskar periwinkle [Catharanthus roseus (L.) G. D] at morphological and biochemical levels. Plant Growth Regulator. 51 : 271-281.

This work is licensed under a Creative Commons Attribution 4.0 International License.





