How to Cite | Publication History | PlumX Article Matrix
Bahador Larti1*, Naser Agh2 and Shahriar Alipour3
1School of Biology, Collage of Science, University of Tehran, Tehran, Iran.
2Artemia & Aquatic Animals Research Institute, Urmia University, Urmia, Iran.
3Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran.20 October 2012.
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1028
ABSTRACT: Artemia spp. as a model organism from Urmia Lake is used in the studies on life history and strategies during hypersalinity conditions. Currently, Urmia Lake water salinity increased to 300 gL-1. In the present study, effects of acending salinity especially hypersaline treatments on survival and growth in three Artemia species namely: (Artemia urmiana, Artemia franciscana and parthenogenetic Artemia) were considered. Six salinity concentrations (50, 80, 150, 200, 250 and 300 gL-1) were applied in this experiment. Survival rate showed that in culture period, survivals were decreased gradually, but in hypersaline treatments (200, 250, 300 gL-1), we were observed dramatic decline in survival parameter. In addition, the mortality rate was calculated for three populations. We were observed high mortality between all salinity treatments in day eight perhaps as a result of larvae phase, but the mortality rate became low until senescent phase, in senescent phase as a final phase; We were expected again high mortality. Growth in three species over time was gradually increased to a constant level, but for each salinity and each population, time to reach a constant level were different. In addition, in hypersaline media (200, 250, 300 gL-1), early maturity were observed for three species.
KEYWORDS: Artemia spp; Ascending Salinity; Survival, Mortality; Growth; Urmia Lake; Iran.
| Copy the following to cite this article: Larti B, Agh N, Alipour S. Survival and Growth of Three Population of Artemia under Laboratory Conditions: Effects of Ascending Salinity Regime. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Larti B, Agh N, Alipour S. Survival and Growth of Three Population of Artemia under Laboratory Conditions: Effects of Ascending Salinity Regime. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=9905 |
Introduction
Although salinity was considered as the ecological niche of Artemia spp. but hypersaline conditions can have major effects on these animals. The genus Artemia (Crustacea: Anostraca) inhabit saline and hypersaline lakes and ponds, which differ from in-water chemistry, seasonality, species composition, and productivity (Lenz and Browne, 1991). A study conducted by Broch (1969) showed that in Artemia, an osmoregulatory mechanism has evolved that involves a change in hemolymph concentration that coincides with changes in water salinity. In addition, Broch, (1969) reported that there is an increase in blood hemoglobin in A.salina, the change in hemoglobin was in direct response to a decrease in oxygen content caused by an increase in salinity. Thus, these physiological adaptations to salinity allow the survival of brine shrimp in their ecological niche. This genus Artemia comprises a complex of sibling species and superspecies defined by a criterion of reproductive isolation (Browne & Bowen 1991). Currently, there are seven species namely: A.salina (A.tunisisana) (In Europe and North Africa); A.franciscana (In New World and Oceania); A.monica (In Mono Lake, USA); A.persimilis (In Argentina); A.urmiana (In Urmia Lake, Iran); A.sinica (In China Asia); and parthenogenetic Artemia (widely distributed throughout the Old World). Additional information on population locations and biogeography is available in Triantaphyllidis et al. (1998). One of the largest permanent water catchments in the West Asia is Urmia Lake. Urmia Lake is a thalassohaline, sodium chloride lake (Leffler, 1961) with oligotrophic characteristics, located at an altitude of 1250 m (Cole and Brown, 1967; Azari-Takami, 1993). Its surface area was reported to range from 4750 to 6100 km2 and the average and greatest depths were 6 and 16 m, respectively (Azari-Takami, 1993; Van Stappen et al., 2001). However, according to recent studies by Agh (2006) the surface area of the lake has reduced to less than 4000 km2 and the average and greatest depths are 3 and 6 m, respectively. Due to drought and increasing demand for agricultural water in the lake’s basin, the salinity of the lake has risen to more than 300 g L-1 during recent years. Urmia Lake has been facing a grave crisis over the past 10 years. Prolonged drought is threatening the lake’s biodiversity and ecology. Reduction of water depth by 6 m, increasing water salinity to saturation level (much higher than tolerance range of Artemia and migrating birds), appearance of huge salt fields around the lake, and huge reduction in Artemia population, is alarming indications of gradual total desiccation of the beautiful and unique ecosystem, the Urmia Lake. It is interesting to know that Artemia is still struggling and fighting against this extreme salinity and one can observe them alive swimming in the lake but information about survival and growth rate in this stressful condition already was unknown. Urmia Lake and neighbouring lagoons are settlements of A.urmiana and parthenogenetic Artemia. A. urmiana was first reported in Urmia Lake by Gunther in 1899. Many other researchers confirmed presence of this bisexual species of Artemia in Urmia Lake (Clark and Bowen, 1976; Barigozzi, 1989; Pador, 1995; Sorgeloos, 1997; Van Stappen et al., 2001; Agh, 2002; Noori and Agh, 2002). Recently Agh et al. (2007) confirmed that a parthenogenetic population of Artemia coexists with the bisexual A. urmiana in Urmia Lake. Parthenogenetic population of Artemia was reported from small lagoons at the vicinity of the Urmia Lake by Agh and Noori (1997). These lagoons are scattered at the periphery of the lake in both West and East Azerbaijan. The size of the lagoons varies from a few square meters to maximum 10000 m surface area and their depth is always less than 0.7 m. Therefore, these lagoons are considered as temporary small water catchments that are dried during early summer and filled up again during winter rains. Water salinity in the lagoon’s ranges from 10-20 g L-1 in early spring and gradually rises to saturation level within about 10 weeks. Parthenogenetic females were observed at high densities with rare males seen only at the ratio of one male to 100 females in these lagoons (Agh and Noori, 1997; Abatzopoulos et al., 2006). There is considerable literature information on survival, growth, morphometry, reproductive and life span characteristics of many bisexual and parthenogenetic Artemia populations (Vanhaecke et al., 1984; Wear and Haslett, 1986; Browne et al., 1984, 1991; Browne and Wanigasekera, 2000; Triantaphyllidis et al., 1995, 1997a, b; Baxevanis et al., 2004; El-Bermawi et al., 2004; Abatzopoulos et al., 2003, 2006b). Most of these studies have contributed to the evaluation of genetic and environmental components of variance in sexual and or clonal Artemia. They have also enabled the comparison of life history characteristics and strategies between different populations (Browne et al., 2002; Abatzopoulos et al., 2003; Baxevanis and Abatzopoulos, 2004; Kappas et al., 2004). However, the effects of salinity on survival and growth of A.urmiana and parthenogenetic populations in these extreme conditions (high salinity) poorly examined. Therefore, salinity may have a significant impact on Artemia characters, especially on survival and growth of Artemia.
For this study, A. urmiana and parthenogenetic Artemia Cysts from Urmia Lake and vicinity lagoons, as well as, for comparison survival and growth of two Artemia population of Urmia Lake, cyst of A.franciscana also hatched in laboratory conditions, and put in different concentrations of salinity (50, 80, 150, 200, 250 and 300 gL-1). Cultured with microalgae as (Dunaliella salina) feeding dietary, and matured in defined period of culturing. The main aim of this study is to present data about role of salinity in adaptations of Urmia Lake Artemia populations with regard as survival and growth factors by statistical analysis.
Materials and methods
Culture procedure: Cysts of each population of Artemia hatched in 35 gL-1 medium under optimal conditions (Sorgeloos et al., 1986). After 16 to 24 hours, depending on species, 500 instars-I nauplii per replicate were directly transferred to cylindroconical flasks and the initial density was 2 nauplii/ml of a culture mediums. In this experiment, six salinity treatments (50, 80, 150, 200, 250, 300 g L-1) were applied. In order to avoid stress, by replacing part of the culture water with medium of higher salinity, the salinity of each flask reached to the desired level, and it was kept constant for the rest of the experiment (Triantaphyllidis et al., 1995). Three replicate at each salinity were used. The animals fed on Dunaliella salina during the period of culture. It can dominate in extreme saline environments with little chance of contamination from other species (Ami Ben-Amotz 2009). The animal density was reduced after day 8 to one individual per 3ml, and after day 14 to one individual per 4ml. The temperature was 28 ± 1 °C and mild aeration were applied from the bottom of the flasks. The photoperiod was 24 h light and fluorescent light tubes were used. In addition, to avoid fluctuations in salinity, salinity of media was continuously monitored. The rate of Artemia survival from each population recorded, in six counting period (8, 11, 14, 17, 20, 23 Days). The first counting period and replacing water was day eight, Artemia in early larval stage are sensitive to water exchange and counting them in early days may cause stress. In addition, for higher salinity treatments, almost at day eight the desired salinity was reached. Total number of surviving Artemia in each population at given salinities, from day 8 to 23 once every three day was recorded (Table, 1). The survival results (expressed as percentages) versus time (expressed as days). Regression analysis (Sokal & Rohlf, 1981) separately performed for each population and salinity. Survival equation according to linear curve estimation calculated. The rate of Artemia mortality from each population recorded, in six counting period (8, 11, 14, 17, 20, 23 Days). Total number of mortality number of Artemia in each population at given salinities, from day 8 to 23 once every three day was recorded (Table, 2). In addition, the mortality percentages (mean value) were plotted against observation times (figure, 4). The total length in each of ten randomly sampled individuals for each population at a given salinity recorded from day 8 to 23 once every three days, according to Triantaphyllidis et al. (1995). The animals immobilized with a logol solution. Total length measured under a dissection microscope fitted with a digitizer. The mean(±SD) of growth rate in six times was recorded (Table, 3). Cluster graph drawn for comparison three populations growth(Figure, 1). The data at a specific time for each population and salinity allowed the calculation of growth rate (K) and Loo using with FISAT-II software with Munro (1982) equation for growth increments. Regression analysis (Sokal & Rohlf, 1981) performed and growth curves according to logarithmic curve estimation were drawn (Figure, 3). Statistical analysis performed within SPSS 16 and FISAT-II.
Results
Survival
Results from survival rate showed that(Table1): The highest survival rate in parthenogenetic Artemia was observed in 50, 150 gL-1 and A.urmiana in 80, 200 and 250 gL-1. At 300 g L-1 (end of counting) survival rate in parthenogenetic Artemia and Artemia urmiana was equal. Almost half of A. franciscana population at 150, 200, 250, 300 gL-1 in the first counting reduced and this population had lower survival compared with others but the maturation rate of the A. franciscana was earlier than others. The
Table 1: Mean values ±SD of survival percentage of three Artemia populations. (PA: parthenogenetic Artemia, A.u: Artemia urmiana, A.f: Artemia franciscana)
| populations | Salinities(gL-1) | Day1 | Day8 | Day11 | Day14 | Day17 | Day20 | Day23 |
| P.A | 50 | 500 | 86.66±5.5 | 82.86±7.06 | 79.4±5.04 | 72.8±7.46 | 67.8±6 | 64.7±7.8 |
| P.A | 80 | 500 | 73.26±1.6 | 64.8±1.31 | 59.46±2.40 | 52.13±2.9 | 45.8±3.6 | 43.3±3.6 |
| P.A | 150 | 500 | 80.00±2.5 | 73.53±1.81 | 65.26±1.61 | 62.0±1.70 | 57.4±1.0 | 52.4±2.1 |
| P.A | 200 | 500 | 61.20±1.4 | 44.93±1.62 | 39.73±1.51 | 34.53±4.0 | 30.9±4.6 | 25.4±4.8 |
| P.A | 250 | 500 | 71.4±4.71 | 58.93±5.70 | 49.73±3.94 | 39.80±8.9 | 35.4±10 | 31.6±9.2 |
| P.A | 300 | 500 | 59.00±3.1 | 54.66±2.40 | 50.06±1.85 | 44.40±1.8 | 40.2±1.3 | 28.0±4.9 |
| A.u | 50 | 500 | 76.8±2.0 | 71.6±2.8 | 68.8±2.15 | 64.0±1.0 | 60.7±1.4 | 55.3±2.4 |
| A.u | 80 | 500 | 86.1±3.8 | 75.6±3.6 | 70.7±4.9 | 63.8±4.3 | 61.0±4.5 | 52.9±4.2 |
| A.u | 150 | 500 | 67.8±6.4 | 61.1±4.6 | 56.9±7.1 | 50.8±7.5 | 46.4±7.6 | 40.6±3.7 |
| A.u | 200 | 500 | 58.4±5.7 | 51.0±3.7 | 46.8±4.41 | 41.6±2.8 | 37.2±4.9 | 32.9±6.4 |
| A.u | 250 | 500 | 74.8±6.9 | 68.0±10.2 | 62.0±9.15 | 52.7±5.9 | 44.3±6.4 | 37.4±3.9 |
| A.u | 300 | 500 | 50.4±5.1 | 46.6±5.0 | 41.6±4.14 | 37.2±5.8 | 33.9±5.7 | 29.8±6.08 |
| A.f | 50 | 500 | 71.4±0.9 | 62.8±2.00 | 60.6±2.01 | 58.4±1.9 | 54.4±2.3 | 50.9±2.05 |
| A.f | 80 | 500 | 69.7±5.5 | 65.6±3.03 | 61.2±2.9 | 57.7±2.8 | 53.8±2.9 | 48.7±1.1 |
| A.f | 150 | 500 | 57.4±3.2 | 51.8±4.13 | 45.4±3.4 | 41.8±1.9 | 37.4±1.1 | 32.8±2.2 |
| A.f | 200 | 500 | 49.0±1.63 | 45.4±1.24 | 43.1±1.8 | 39.6±2.4 | 35.0±4.7 | 32.2±4.4 |
| A.f | 250 | 500 | 46.4±2.01 | 43.2±1.13 | 39.8±1.11 | 36.6±1.4 | 27.5±3.2 | 21.9±1.8 |
| A.f | 300 | 500 | 46.1±3.71 | 41.2±3.10 | 35.2±3.4 | 26.5±1.6 | 18.0±2.0 | 14.4±1.7 |
first signs of puberty in parthenogenetic Artemia were at salinity and culture days as following: at 50 gL-1 30th day, 80 gL-1 25th day, 150 gL-1 19th day, 200 gL-1 15th day, 250 gL-1 14th day and 300 gL-1 12th culture day. In A.urmiana these were 50 gL-1 28th day, 80 gL-1 23th day, 150 gL-1 21th day, 200 gL-1 18th day, 250 gL-1 18th and 300 gL-1 15th of culture day. In A.franciscana these values were 50 gL-1 18th day, 80 gL-1 15th day, 150 gL-1 10th day, 200 gL-1 9th day, 250 gL-1 9th and 300 gL-1 9th day. The survival cluster graph (Figure, 1) shows that parthenogenetic Artemia at 50, 150 and 300 gL-1 had higher survival, while A.urmiana at 80, 200, 250 gL-1 had higher survival than other two species. Overall, survival rate decreased gradually with increasing salinity. While, we were obsearved high Mortality between all salinity treatments in day 8 perhaps as a result of larvae phase, but mortality rate became low untile senescent phase, in senescent phase as a final phase, we could see in some treatments again, high mortality in the last counting period.
 |
Figure 1: Survival of the three different Artemia populations reared at six salinities (50, 80, 150, 200, 250 and 300 gL-1).
|
For each population and salinity. A: A.urmiana, B: A.franciscana, C: parthenogenetic Artemia. In graphs: A.urmiana (ـــــــ, Quadrate), A.franciscana (ــــ ـــــ , Triangle) and parthenogenetic Artemia (ـ ـ ـ ـ, Circle).
Growth
Growth curves in different salinity treatments were in accordance with the logarithmic model (Fig 2). Growth in all salinity increases gradually during the measurement period increased to a constant level, but for each salinity and each population, time to reach a constant level were different. The growth rates in A. urmiana at all salinity treatments are higher than the other two populations (Table, 2).
Table 2: Mean values ±SD of Growth of three Artemia populations. (PA: parthenogenetic Artemia, A.u: Artemia urmiana, A.f: Artemia franciscana)
| populations | Salinities(gL-1) | Day8 | Day11 | Day14 | Day17 | Day20 | Day23 |
| P.A | 50 | 3.0±0.16 | 3.5±0.45 | 5.4±0.05 | 6.2±0.51 | 6.4±0.62 | 7.0±0.49 |
| P.A | 80 | 3.7±0.26 | 4.5±0.38 | 6.2±0.12 | 6.6±0.42 | 7.5±0.58 | 8.2±0.64 |
| P.A | 150 | 4.2±0.48 | 4.5±0.42 | 6.1±0.80 | 6.8±0.67 | 7.3±0.71 | 7.6±1.11 |
| P.A | 200 | 3.9±0.26 | 4.7±0.41 | 6.7±0.22 | 7.4±0.81 | 7.8±0.75 | 8.1±1.2 |
| P.A | 250 | 3.7±0.24 | 5.8±0.45 | 7.0±0.36 | 7.4±0.35 | 7.8±0.37 | 8.1±0.18 |
| P.A | 300 | 3.6±0.18 | 5.4±0.77 | 6.5±0.38 | 6.5±0.38 | 6.6±0.47 | 6.7±0.46 |
| A.u | 50 | 5.0±0.72 | 7.1±0.65 | 8.2±0.35 | 9.1±0.5 | 10.6±0.9 | 11.6±1.15 |
| A.u | 80 | 5.6±0.94 | 7.9±0.3 | 8.7±0.51 | 9.7±0.53 | 10.8±0.8 | 11.9±0.45 |
| A.u | 150 | 4.4±0.45 | 7.2±0.38 | 9.7±0.5 | 10.2±0.19 | 10.4±1.0 | 11.2±1.70 |
| A.u | 200 | 4.4±0.6 | 6.5±0.24 | 9.0±2.04 | 10.7±1.16 | 11.1±1.8 | 11.5±0.42 |
| A.u | 250 | 4.4±0.49 | 6.9±0.25 | 7.7±0.51 | 9.0±0.81 | 9.5±1.33 | 9.8±0.62 |
| A.u | 300 | 3.5±0.25 | 6.3±0.63 | 6.9±0.16 | 8.4±0.55 | 9.2±0.51 | 9.4±0.08 |
| A.f | 50 | 4.5±0.45 | 5.6±0.18 | 6.5±0.25 | 7.0±0.06 | 8.0±0.11 | 8.3±0.13 |
| A.f | 80 | 4.7±0.67 | 5.6±0.41 | 6.1±0.28 | 6.3±0.39 | 6.8±0.47 | 6.9±0.49 |
| A.f | 150 | 5.2±0.22 | 6.3±0.74 | 6.5±0.61 | 7.5±0.31 | 7.5±0.3 | 7.8±0.33 |
| A.f | 200 | 5.3±0.05 | 6.4±0.2 | 6.6±0.17 | 6.8±0.30 | 7.3±0.16 | 8.4±0.62 |
| A.f | 250 | 5.0±0.36 | 6.5±0.22 | 6.7±0.21 | 6.9±0.19 | 7.2±0.41 | 7.9±0.59 |
| A.f | 300 | 5.2±0.28 | 5.8±0.39 | 6.6±0.31 | 6.7±0.36 | 7.2±0.32 | 7.8±0.50 |
However, in all salinity measurements at day eight the A.franciscana growth rate was higher than the other two populations due to maturation rate of the A.franciscana, which was earlier than other two populations. In addition, comparison of growth rates of three populations, showed that difference between populations. The total length of parthenogenetic Artemia and A. franciscana approximately was equal.
 |
Figure 2: Growth of the three different Artemia populations reared at six salinities (50, 80, 150, 200, 250 and 300 gL-1). For each population and salinity. |
For each population and salinity. A: A.urmiana, B: A.franciscana, C: parthenogenetic Artemia. In graphs: A.urmiana (ـــــــ, Quadrate), A.franciscana (ــــ ـــــ , Triangle) and parthenogenetic Artemia (ـ ـ ـ ـ, Circle).
In addition, at hypersaline treatments like 150, 200, 250 and 300 gL-1 growth stopped earlier. According to K and Loo values A. urmiana at 80 and 150 gL-1 had maximum growth rate and at 300 gL-1 had minimum one, while parthenogenetic Artemia and A. franciscana at 200 and 250 gL1 had maximum growth rates (Fig2). Adaptation to stress condition was observed in A. franciscana because of this population in 250, and 300 gL-1 showed that gradually increasing growth rate than other populations.Also, Cluster graph (Figure, 3) shows growth, survival and mortality between populations and according to data, A. franciscana at 50, 150, 250 and 300 gL-1 had higher mortality.
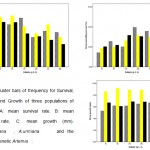 |
Figure3: cluster bars of frequency for Survival, Mortality and Growth of three populations of Artemia. |
A: mean survival rate, B: mean mortality rate, C: mean growth (mm). A.franciscana , A.urmiana and the parthenogenetic Artemia .
Discussion
Optimum salinity for Artemia is favorable condition for living. But according to Post and Youssef (1977) Artemia can survive in nature even at 340 gL-1. Except Salinity as the main factor, temperature and feeding in natural environment are important factors influencing Artemia populations (Wear and Haslett, 1987). The survival at high salinities, according to Triantaphyllidis et al. (1995) might be related to the Artemia osmoregulatory system function. Artemia culture and maintenance in laboratory at salinities higher than 200 gL-1 has always been difficult (Wear and Haslett, 1986; Wear et al., 1986). Browne and Hoopes (1990) reported only 9% survival at 190 gL-1 and no survival at all at 230 gL-1 in a parthenogenetic Artemia from Salin de Giraud (France). Dana and Lenz (1986) studying the bisexual Artemia from Mono Lake, California, USA, found low survival in 159 and 179 gL-1 under laboratory conditions. Triantaphyllidis et al. (1995) reported over 80% mortality of both parthenogenetic Artemia from Tanggu area (China) and A. franciscana at 180 gL-1 at 25°C in 23 days culture period. On the contrary, they reported above 75% survival for A. franciscana and higher than 50% survival for parthenogenetic Artemia at salinities lower than 100 gL-1. In the experiments performed by El-Bermawi et al. (2004) on Artemia populations from Egypt, 100% mortality was observed in bisexual A. salina from Wadi El-Natrun in 150 and 200 gL-1 within 17 days, but the high salinity had little effect on the ability of parthenogenetic populations to survive. In this experiment six salinity treatments (50, 80, 150, 200, 250, 300 gL-1) were used. Total mortality did not occur in our experiments for either A.franciscana and A.urmiana or parthenogenetic Artemia within the range of 50-250 gL-1 salinities. However, under treatment 300 gL-1, in all of the populations, no survival until day 37 to 40 was recorded. Browne and Wanigasekera (2000) observed an increase in survival of parthenogenetic Artemia from Margherita di Savoia (Italy) and A. salina when salinity of the culture medium was increased from 60 to 120 gL–1 at 15°C, but this percentage sharply decreased in three other bisexual species including A. sinica, A. franciscana and A. persimilisi. Inversely, at 24°C they got completely different results, obtaining significantly higher survival at higher salinity. El-Berrnawi et al. (2004) observed similar results with parthenogenetic Artemia populations from Egypt. Contrary to these two findings, Triantaphyllidis et al. (1995) find a steady decrease in survival in both parthenogenetic from Tanggu (China) and bisexual A. franciscana cultured in the range of 60 to 180 g L -1. Triantaphyllidis et al. (1995) reported 70-80% survival for A.franciscana at 60 g L -1, but Browne and Wanigasekera (2000) observed only 16% survival for this species at the same salinity. Vanhaecke et al. (1984) reported high survival for A. sinica and A. salina at 60 g L -1, whereas survival was zero for these two species at the same salinity in the experiments performed by Browne and Wanigasekera (2000). Browne and Wanigasekera (2000) claimed that differences in the culture conditions and intra-species and population-dependent characteristics could be among the reasons for the different results obtained by different researchers. Agh et al. (2008) mentioned that mortality of 100% was observed in bisexual populations reared at salinities from 150 to 200 g L-1. The results of present agree with the findings of Triantaphyllidis et al. (1995), showing a constant decline in survival when salinity increases from 50 to 300 gL-1. Our experiments showed that A.franciscana was well adapted to given salinities, as this species produce more nauplii at salinity 250 gL-1 than other two populations. Presented data showed that parthenogenetic Artemia had higher survival at 50, 150 gL-1 while A.urmiana at 80, 250 gL-1 and A.franciscana (in the end of counting period) at salinity 250 gL-1 had higher survival rate. Abatzopoulos et al. (2006b) reported very low survival for A. urmiana at salinities of 35 and 50 g L -1. However, they found high survival at 100, 140 and 180 g L-1.
Present results are opposite to findings of Abatzopoulos et al. (2006b). In the present study, survival percentages of both sexual and asexual populations were found to decrease with increasing salinity. According to the results obtained in present study, tolerance of A.urmiana and A.franciscana at salinity 200 gL-1 resulted in higher survival rate than parthenogenetic Artemia but inversely parthenogenetic Artemia in the end of counting period at salinity 300 gL-1 had survival rate equal to A.urmiana and at salinity 250 gL-1 this population showed higher survival rate than A.franciscana. However, high mortality was observed in A.franciscana at salinity 50, 150, 250 and 300 gL-1, whereas parthenogenetic populations showed sharp mortality at 80 and 200 gL-1. Stress had significant effect on growth rate at different salinities (see K values, fig4) in all three populations, and current experiment became in agreement with results of Gilchrist (1960), Triantaphyllidis et al. (1995) and El-Bermawi et al. (2004). They showed growth is inversely related to salinity. Triantaphyllidis et al. (1995) reported significant differences in the growth of Artemia especially in the parthenogenetic population from Tanggu (China) cultured at different salinities. According to their experiments maximum growth in A. franciscana was observed at 35 gL-1, whereas growth in parthenogenetic Artemia showed no differences in 35, 60 and 100 gL-1. However, parthenogenetic Artemia at 180 gL-1 attained only 50% of the length of those at 35, 60 and 100 gL-1. A.franciscana at 180 gL-1 achieved 60% of the length in comparison to animals grown at 35 gL-1. El-Bermawi et al. (2004) observe no significant differences in growth of parthenogenetic and bisexual populations of Artemia from Egypt reared in the laboratory at salinities ranging from 35 to 200 gL-1. Abatzopoulos et al. (2006a) find that growth rate of A. urmiana was not affected by the increase of salinity. In the present experiment, A.urmiana had higher growth rate than two other populations. In A.urmiana at salinity 80 gL-1, maximal growth rate was achieved. In A.urmiana salinity 50 and 80 gL-1, salinity 150 and 200 gL-1 , salinity 250 and 300 gL-1 showed relatively equal growth rate. A. franciscana and parthenogenetic Artemia had relatively equal length in all salinity treatments. In hypersaline treatments increasing phase of growth, reached to a constant level faster than low salinities. In present study, we used Urmia Lake water adjusted to 50, 80, 150, 200, 250 and 300 gL-1throughout the experiment, whereas Abatzopoulos et al. (2006b) used artificially prepared D and K medium of 35, 50, 100, 140 and 180 g L -1 salinity in their experiments. Triantaphyllidis et al. (1995) reported significant differences in parthenogenetic Artemia growth in Tanggu (China) populations cultured at different salinities. These authors reported that a maximum growth of A. franciscana was observed at 35g/l (10.16±0.85mm) and they observed, these species experienced 100% mortality at 40g/l salinity, and showed better growth at 120g/l (9.269±0.263mm). In the present experiment for A.urmiana population at salinity 80 gL-1 (11.9±0.45), for A.franciscana at salinity 200 gL-1 (8.4±0.62) and for parthenogenetic Artemia at salinity 80 gL-1 maximum growth rate achieved (8.2±0.64) until day 23. Present results suggest that adaptation to different salinities are species-specific, and different salinities dependent on the culture conditions and feeding regime, can have different effects on survival, mortality and growth rates.
Acknowledgements
We thank Mr Eshghi for assistance with laboratory work. Special thanks from Mr Salehi in Tehran university laboratory.
References
- Abatzopoulos T.J, El-Bermawi N, Vasdekis C, Baxevanis AD, and Sorgeloos P. Effects of salinity and temperature on reproductive and life span characteristics of clonal Artemia. International Study onArtemia, LXVI. Hydrobiologia, 492: 191-199, 2003.
- Abatzopoulos T.J, Agh N, Van Stappen G, Razavi Rouhani S.M, and Sorgeloos P. Artemia sites in Iran. J. Mar. Biol, Assoc. United Kingdom, 86: 299-307, 2006a.
- Abatzopoulos T.J, Baxevanis AD, Triantaphyllidis G.Y, Criel G, Pador E.L, Van Stappen G, et al. Quality evaluation of Artemia urmiana Gunther (Urmia Lake, Iran) with special emphasis on its particular cyst characteristics. International Study on Artemia, LXIX Aquaculture, 254: 442-454, 2006b.
- Agh N, and Noori F. Introduction of a parthenogenetic population of Artemia from lagoons around Urmia Lake and its morphological comparison with Artemia urmiana. – In: MOELLEM, T. (ed.), Proceedings of the First Iranian Congress of Zoology. University of Teacher Education, 17–18 September 1997.
- Agh N. Scientific report on resource assessment of Artemia in Lake Urmia. Artemia and Aquatic Animals Research Center, Urmia University, pp: 150, 2006.
- Agh N, Abatzopoulos T.J, Van Stappen G, Razavi Rouhani S.M, and Sorgeloos P. Coexistence of sexual and parthenogenetic Artemia populations in lake Urmia and neighbouring Lagoons. Int. Rev. Hydrobiol., 1: 48-60, 2007.
- Agh N, Vanstappen G, Bossier P, Sepehri H, Lotfi V, Razavi Rouhani SM, et al. Effects of salinity on survival, Growth, Reproductive & life span characteristics of Artemia populations from Urmia Lake & neighboring lagoons. Pakistan Jornal of Biological Sciences, 11 (2): 164-172, 2008.
- Ami Ben-Amotz J. E, Subba Rao W. P. The Alga Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology. Enfield, Science Publishers, 2009.
- Azari-Takami G. Urmia Lake as a valuable source of Artemia for feeding sturgeon fry. J. Vet. Fac. Univ., Tehran, 47: 2-14, 1993.
- Barigozzi C. The problem of Artemia urmiana. Artemia Newslett., 14: 4, 1989.
- Baxevanis AD. and Abatzopoulos T.J. The phenotypic response of ME2 (M. Embolon, Greece) Artemia clone to salinity and temperature. J. Biol, Res., 1: 107-114, 2004.
- Broch E. S. The osmotic adaptation of the fairy shrimp Branchinecta campestris Lynch to saline astatic waters. Limnology and Oceanography. 14: 485-492, 1969.
- Browne, RA, Salee S.E., Grosch D.S., Segreti w.o. and Purser S.M. Partitioning genetics and environmental components of reproduction and life span inArtemia.Ecology, 65: 949 969, 1984.
- Browne, RA and Hoops C.W. Genotype diversity and selection in asexual brine shrimp (Artemia). Evolution, 44: 1035-1051,1990.
- Browne R.A and Bowen S.T. Taxonomy and Population Genetics of Artemia. In: Artemia Biology, Browne, RA, P. Sorgeloos and C.N.A Trotman (Eds.). CRC Press. Boca Raton, Florida, USA, pp: 221-235,1991.
- Browne R.A, Li M, Wanigasekera G, Simonek S, Brownlee D, Eiband G.and Cowan J. Ecological, physiological and genetic divergence of sexual and asexual (diploid and polyploid) brine shrimp (Artemia). Adv. EcoI., 1: 41-52, 1991.
- Browne, RA and Wanigasekera G. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. J. Exp. Mar. BioI. EcoI., 244: 29-44, 2000.
- Browne R.A, Moller V, Forbes V.E, and Depledge M.H. Estimating genetic and environmental components of variance using sexual and clonal Artemia. I. Exp. Mar. BioI. EcoI., 267: 107-119, 2002.
- Clark L.S, and Bowen S.T. The genetics of Artemia salina. VII. Reproductive isolation. J. Hered., 67: 385-388, 1976.
- Cole G.A, and Brown R J. The chemistry of Artemia habitats. Ecology, 48: 858-861, 1967.
- Dana G.L, and Lenz P.H. Effects of increasing salinity on an Artemia population from Mono lake, California. Oecologia, 68: 428-436,1986.
- El-Berrnawi N, Baxevanis AD, Abatzopoulos TJ, Van Stappen G and Sorgeloos P. Salinity effects on survival, growth and morphometry of four Egyptian Artemia populations (International Study on Artemia. LXVII). Hydrobiologia, 523: 175-188, 2004.
- Gilchrist B.M. Growth and form of the brine shrimp Artemia salina L. Proc. Zool, Soc. Lond., 134: 221-235, 1960.
- Günther R.T. Contributions to the geography of Lake Urmi and its neighbourhood. Geogrraphy J., 14: 504-523, 1899.
- Kappas l, Abatzopoulos TJ, Hoa NY, Sorgeloos P, and Beardmore J.A. Genetic and reproductive differentiation of Artemia franciscana in a new environment. Mar. BioI., 146: 103-117, 2004.
- Leffler H. Contributions to the knowledge of Iranian inland waters II. Regional limnological study with special focus on the Crustacean fauna. Int. Rev. Hydrobiol., 46:309 406, 1961.
- Lenz P. and Browne R. Ecology of Artemia. In Browne, R. A. and Sorgeloos, P. (eds), Artemia Biology. C. N. A. Trotman, CRC Press, Ch 10, pp. 237–254, 1991.
- Munro J.L. Estimation of the parameters of the von Bertalanffy growth equation from recapture data at variable time intervals. J. Cons. CIEM, 40: 199-200, 1982.
- Pador E. Characterization of Artemia urmiana Gunther 1900 from Lake Urmia, Iran, M. Sc. Thesis, Vrije Universiteit Brussel and Laboratory of Aquaculture-Artemia Reference Center, University of Ghent, Belgium, 1995.
- Post F.J, and Youssef N.N. A prokaryotic intracellular symbiont of the Great Salt Lake brine shrimp Artemia salina L. Can. J. Microbiol., 23: 1232-1236, 1977.
- Sokal R, and Rohlf J.F. Biometry. W. H. Freeman and Company, San Francisco, California, USA, 859 pp, 1981.
- Sorgeloos P, Lavens P, Leger P.H, Tackaert W, and Versichele D. Manual for the culture and use of brine shrimp Artemia in aquaculture. Laboratory of Mariculture, State University of Ghent, Belgium, pp: 319, 1986.
- Sorgeloos P. Lake Urmia cooperation projectcontract item A. Report on the Determination and Identification of Biological Characteristics of Artemia urmiana for Application in Aquaculture. Faculty of Agriculture and Applied Biological Science, Laboratory of Aquaculture and Artemia Reference Center, Ghent University, Belgium, pp: 6-16, 1997.
- Triantaphyllidis G. V, Poulopoulou K, Abatzopoulos T. J, Pinto Perez C. A. and Sorgeloos, P.. International Study on Artemia XLIX. Salinity effects on survival, maturity, growth, biometrics, reproductive and life span characteristics of a bisexual and parthenogenetic populations of Artemia. Int. J. Salt Lake Res., 8, 267–285, 1995.
- Triantaphyllidis G.V, Abatzopoulos T.J, and Sorgeloos P. Review of the biogeography of the genus Artemia (Crustacea, Anostraca). J. Biogeogr. 25: 213-226, 1998.
- Vanhaecke P, Siddal S.E, and Sorgeloos P. Combined effects of temperature and salinity on the survival of Artemia of various geographical origin. International study on Artemia. J. Exp. Mar. Biol, EcoI., 98: 167-183, 1984.
- Van Stappen G, Fayazi G, and Sorgeloos P. Field study of the Artemia urmiana (Gunter, 1890) population in Lake Urmia, Iran. International Study on Artemia. Hydrobiologia, 466: 133-143, 2001.
- Wear R.G, and Haslett S.I. Effects of temperature and salinity on the biology of Artemia franciscana Kellogg from lake Grassmere, New Zealand. 1. Growth and mortality. J. Exp. Mar. BioI. EcoI., 98: 153-166, 1986.
- Wear R. G, Haslett S. J, and Alexander N. L. Effects of temperature and salinity on the biology of Artemia franciscana Kellogg from Lake Grassmere, New Zealand. 2. Maturation, fecundity and generation times. J. Exp. Mar. Biol. Ecol., Vol. 98, pp. 167-183, 1986.
- Wear R.G, and Haslett S. J. Studies on the biology and ecology of Artemia from Lake Grassmere, New Zealand. In, Artemia research and its applications, Vol. 3, edited by P. Sorgeloos et al., Universa Press, Wetteren, Belgium, pp. 101-126, 1987.

This work is licensed under a Creative Commons Attribution 4.0 International License.





