How to Cite | Publication History | PlumX Article Matrix
Synthesis of Silver Nanoparticles from Techoma Stains Flower Extract Towards Flower Utilization
K. Vijaya Sudhakar, D. Srinivasa Rao* and K.R.S. Sambasiva Rao
Department of Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar - Guntur - 522 510, India.
Corresponding Author E-mail: krssrao@yahoo.com
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1078
ABSTRACT:
Silver nanoparticles synthesis of varying sizes using Techoma stains flower extract at room temperature. The synthesized silver nanoparticles are characterized by using UV-visible spectrophotometer and Transmission electron microscope (TEM). The effect of the time on the particle size has been reported. Synthesis of flower extract mediated silver nanoparticle is very cost effective and eco friendly and therefore can be economic and effective alternative for the large scale synthesis of silver nanoparticles.
KEYWORDS: Biographical notes.
| Copy the following to cite this article: Sudhakar K. V, Rao D. S, Rao K. R. S. S. Synthesis of Silver Nanoparticles from Techoma Stains Flower Extract Towards Flower Utilization. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Sudhakar K. V, Rao D. S, Rao K. R. S. S. Synthesis of Silver Nanoparticles from Techoma Stains Flower Extract Towards Flower Utilization. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10204 |
Introduction
Now a days the Nanoparticles shows the evidence for completely novel or better properties based on particular characteristics features such as size, delivery and morphology. These nanoparticles and Nanomaterials illustrate the novel applications flourishing rapidly [1,2,3]. These nanomaterials often show unique and considerably changed their physical, chemical and biological properties compared to their macro scaled counterpart[4]. Though, it is well known that inorganic nanomaterials are good antimicrobial agents. In progress research the bactericidal nanomaterials has opened a new era in pharmaceutical industries. These silver nanoparticles are the metal of choice as they hold the promise to kill microbe’s effectively [5]. The most important application of silver and silver nanoparticles is in topical ointments to prevent infection against burn and open wounds [6]. Silver has been documented to have inhibitory action on microbes present in medical and industrial process [7,8]. Recently metal nanoparticles reported to be good carrier for targeted delivery [9].
Nanoparticles can be prepared from a variety of materials such as proteins, polysaccharides and synthetic polymers. The selection of matrix materials is dependent on many factors including size of nanoparticles required ,inherent properties of the drug, e.g. aqueous solubility and stability, surface characteristics such as charge and permeability, degree of biodegradability, biocompatibility and toxicity, drug release profile desired and antigenicity of the final product.(10)Chemical reduction is the most frequently applied method for the preparation of silver nanoparticles (Ag NPs) as stable, colloidal dispersions in water or organic solvents.(11,12).
Techoma stain is an erect shrub or small tree up to 4 m tall. Leaves are opposite and imparipinnate. In India, it is known as Sonapatti in Tamil language, Pachagotla in Telugu language and korenekalar in Kannada language (13). Yellow elder has been used for a variety of purposes in herbal medicine. Some drugs of plant origin in conventional medical practice are not pure compounds but direct extracts or plant materials that have been suitably prepared and standardized (14).
However, here we report synthesis of silver nanoparticles, reducing silver ions present in the aqueous solution of silver nitrate complex by the extract of Techoma stains flowers. And the morphological characterizations are performed using Transmission electron microscope (TEM) and FTIR. The optical absorption properties are measured using UV-visible spectrophotometer and observed the absorption peaks in 419-420 nm regions, which are close to the characteristics surface plasmon resonance (SPR) wavelength of metallic silver.
Experimental Procedure
Preparation of Techoma stains flower extract
Techoma stains flower extract have been prepared by bringing the fresh flowers of Techoma stains from our campus to the Laboratory. Fresh petals weighing 15 gm are at first thoroughly washed several times in distilled water, cut into fine pieces and then boiled in a 500ml beaker with 150ml of distilled water up to 10 min and then filtered to separate out the broth.
Synthesis of silver nanoparticles
Aqueous solution of silver nitrate (AgNO3, Fischer, India) of 0.5mM is prepared in a 250 ml beaker and the solution is added to the flower extract solution at room temperature. The color change in the colloidal solutions occurred indicating the formation of silver nanoparticles. Then two sets of sample are collected, one at room temperature and another 3 and 6 minutes of reduction time. Two samples were synthesized at room temperature maintaining at different intervals of time for 3 minutes of reaction, and another sample was synthesized after 6 minutes of reaction at room temperature.
UV-Vis Spectra analysis
The reduction of Ag+ ions was observed by measuring the UV-Vis spectrum of the reaction medium at two different intervals time after diluting a small aliquot of 100 μL of the sample with 1 ml deionized water. UV-Vis spectral analysis has been done by using a UV-Vis Jasco-550
Transmission electron microscope analysis
Transmission Electron Microscopic (TEM) analysis was done using a Tecnai-12 FEI, Netherlands in IICT Hyderabad. Thin film of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the TEM grid were allowed to dry by putting it under a mercury lamp for 5 min.
 |
Figure 1.a: Silver nitrate Solution (1 mM) left (before) and right (after) addition of flower extract solutions.
|
 |
Figure 1. b:UV-Vis. Absorption spectra recorded as a function of time of reaction of 1:1Solution of silver ions by Techoma stains flower extract in the range 419 nm after 10Minutes reaction.
|
Figure 1 and 2 showed that absorption peaks of UV spectroscopy at 400-420 nm maximum were observed it may indicate formation of heparin silver nanoparticles. The generation of heparin silver nanoparticles can be identified UV-Vis spectroscopy where the characteristic of the surface Plasmon resonance (SPR) bands are found at 420 nm at absorbance of 0.081. The maximum absorption may be due to the formation of silver nanoparticles. The position of the peak and shape of the Plasmon absorption band are closely related to the size, shape and dispersion appearance of the silver nanoparticles formation. Therefore, the change in the plasmon absorption of silver nanoparticles indicated that the size of the heparin silver nanoparticles and altered with the concentrations of heparin, which acted as a controller of nucleation as well as a stabilizer.
Transmission electron microscope analysis of Silver Nanoparticles
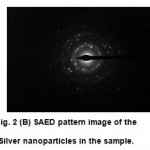 |
Figure 2 (A): TEM Micrograph of the sample after the 10 minute reaction kinetics with treating flower extract with silver ions complex(1mM) in the ratio of 1:1, showing particles of irregular shapes which varies in size from 30 nm to 80 nm (average particle size is50nm).
|
 |
Figure 2 (B): SAED pattern image of the Silver nanoparticles in the sample.
|
Results
Absorption spectroscopy is an analytical technique used to determine the identities and quantities of substances. The technique is based upon the interaction of electromagnetic radiation with matter. Electrons in atoms or molecules can be raised, or “excited”, from one energy state to another by the absorption of electromagnetic radiation. The transition of an electron from one energy state to another is permitted only if the energy of the radiation is equal to the energy difference between the two states.
Fig.1shows that the optical photograph of the color change in the colloidal solution of nanoparticles reduced by Techoma (Fig.1)flower extract with time (in the inset). UV-Vis spectrograph of the sample solution of silver nanoparticles has been recorded as a function of time. Absorption spectra of silver nanoparticles formed in the reaction media at 30min. has absorbance peak at 420 nm, The UV-Vis spectroscopy revealed the formation of silver nanopartícles by exhibing the typical surface Plasmon absorption maxima at 420 nm the UV–Vis spectrum which implicates monodisperse Ag nanoparticles. The silver Plasmon band shifts to a higher wavelength with increasing reducing agent concentration using UV-Vis spectroscopy shows that the silver colloids formed are nanosized, uniformly distributed.TEM Micrograph Fig.2a of the silver nanoparticles synthesized after10 min of the reaction having irregular shapes of 20 to 80 nm with average size 50 nm. The selected area electron diffraction (SAED) pattern of the nanoparticles in Fig.2b shows face centered cubic crystalline structure of silver with indexed different diffracting planes.
Discussion
Reduction of silver ions present in the aqueous solution of silver complex during the reaction with the ingredients present in the plant flower extract observed by the UV-Vis spectroscopy revealed that silver nanoparticles in the solution may be correlated with the UV-Vis spectra. As the Techoma flower extract was mixed in the aqueous solution of the silver ion complex, it started to change color from water color to yellowish brown (Fig.1); color was changed due to excitation of surface Plasmon vibrations, which indicated formation of silver nanoparticles. UV-Vis spectroscopy is well known to investigate shape and size controlled of nanoparticles. UV-Vis spectrograph of the solution of silver nanoparticles has been recorded as a function of time by using a quartz cuvette with water as reference, repeated experiments were carried out with varying the amount of silver ion complex (1mM) and flower extract it was observed that precursors in the ratio of 1:1 gave best results of our interest. It is interesting to note that most of the particles 50nm in the TEM pictures are not in physical contact but are separated by a fairly uniform interparticle distance. The sharp spots in the SAED pattern (Fig. 2b) indicate that the silver nanoparticles are single crystalline in nature.
Conclusion
Here we have reported, the synthesis of silver nanoparticles of varying sizes using Techoma stains flower extract in the aqueous solution at room temperature. The variation of particle size with the reaction temperature and reaction time has been reported. The structural characterizations of the samples are performed using TEM analysis. The UV-visible optical absorption properties are measured and found the shift of Surface Plasmon Resonance wavelengths with the average particle size of the synthesized samples. This green chemistry approach towards the synthesis of silver nanoparticles has many advantages. Flower extract is being eco friendly and very cost effective; the presented method can be economic and effective alternative for the large scale synthesis of silver nanoparticles in nanotechnology processing industries.
References
- W. Jahn J. Struct. Biol. 127, 106 (1999).
- H. S. Naiwa Ed. HandBook of Nanostructural Materials and Nanotechnology Academic Press New York 1-5.(2000).
- C. J. Murphy, J. Mater Chem. 18, 2173–2176 (2008).
- Li L, Hu J, Alivistos AP. Nano Lett 2001;1:349.
- I. Sondi, B. Salopek-Sondi J Colloid Interface Sci. 275, 177 (2004).
- M. Ip, S. L. Lui, V. K. M. Poon, I. Lung, A.Burd J. Medical Microbiol. 55, 59–63(2006).
- R. M. Jose, L. E. Jose, C. Alejandra, Nanotechnology 16, 2346–2353 (2005).
- C. Lok, C. Ho, R. Chen, Q. He, W. Yu, H.Sun, P. K. Tam, J. Chiu, C. Che J. Biol.Inorg. Chem. 12, 527–534 (2007).
- Bhumkar, D.R., Joshi, H.M., Sastry, M., and Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharmaceut Res., 2007, 24, 1115–1126.
- Kreuter J. Nanoparticles. In Colloidal drug delivery systems, J, K., Ed. Marcel Dekker: New York,1994; pp 219-342.
- Tao A, Sinsermsuksaku P, Yang P. Angew Chem Int Ed 2006;45:4597
- Wiley B, Sun Y, Mayers B, Xi Y. Chem-Eur J 2005;11:454.
- Parrotta J A., Healing plants of Peninsular India, CABI publishing, 2001, 701-702.
- Donald E C.,Medicinal plant research in Nigeria:Retrospect and Prospect. Inofowora (Ed). The state of medicinal plants research in Nigeria,Nig. Soc. Of Pharmacognosy, Ibadan University press, Nigeria,1986,1 – 12.
- World Health Organisation (WHO). Antimalarials Drug Combination Therapy. Report of a WHO Technical Consultation. April,2001, 1 -2.
- World Health Organisation (WHO). WHO urges Countries to act on New Anti – Resistance Malaria Medicines. Press release WHO/31, April 2002, 1 -3.

This work is licensed under a Creative Commons Attribution 4.0 International License.





