How to Cite | Publication History | PlumX Article Matrix
K. Perinbam1, S. Arokiyaraj1, Alex Mathew1, O.S. Aysha2, K. Balaraju3 and P. Agastian3
1PG and Research, Department of Biomedical Engineering, Sathyabama University, Chennai - 119 India.
2Elite Bioscience Foundation, Chennai - 119 India.
3Department of Plant Biology and Biotechnology, School of Life Sciences, Loyola College, Chennai - 34 India.
Corresponding Author E-mail: sistperinbam@yahoo.com
ABSTRACT: The present study was carried out to evaluate the immunomodulatory activity of four Indian medicinal plants. Solanum nigrum, Indigofera tinctoria, Euphorbia hirta and Azima tetracantha were tested for stimulation and inhibition of lymphocyte proliferation via lymphocyte proliferation assay by 3H thymidine uptake. RT-PCR was investigated to detect in vitro induction of cytokines such as IL-4 and IFN-g for all the extracts. Among the plants tested Azima tetracantha (100 μg/ml) showed the highest rate of lymphocyte proliferation (76%), Indigofera tinctoria (100 μg/ml) showed maximum inhibition of lymphocyte proliferation (70%). A. tetracantha and S. nigrum showed IFN- g induction while I. tinctoria and A. tetracantha showed IL-4 induction. This in vitro study suggests that A. tetracantha and I. tinctoria are potent to have immunomodulatory agents.
KEYWORDS: Immunomodulation; Lymphocyte proliferation; RT-PCR; Vero cell
Download this article as:| Copy the following to cite this article: Perinbam K, Arokiyaraj S, Mathew A, Aysha O. S, Balaraju K, Agastian P. Immunomodulatory properties of Indian medicinal plants in Kolli hills: Stimulation and suppression of lymphocyte proliferation - In vitro. Biosci Biotechnol Res Asia 2008;5(1) |
| Copy the following to cite this URL: Perinbam K, Arokiyaraj S, Mathew A, Aysha O. S, Balaraju K, Agastian P. Immunomodulatory properties of Indian medicinal plants in Kolli hills: Stimulation and suppression of lymphocyte proliferation - In vitro. Biosci Biotechnol Res Asia 2008;5(1). Available from: https://www.biotech-asia.org/?p=6584 |
Introduction
Medicinal plants which form the backbone of traditional medicine, have in the last few decades been the subject for very intense pharmacological studies; this has been brought about by the acknowledgement of the value of medicinal plants as potential sources of new compounds of therapeutics value and as a sources of lead compounds in the drug development [1]. Medicinal plants are commonly used for the treatment of various ailments, as they are considered to have advantages over the conventionally used drugs that are expensive and known to have harmful side effects. Since, there is an ever-growing interest in investigating different species of plants to identify their potential therapeutic applications. This increasing interest is due to a tremendous historical legacy in folk medicine use of plants as medicine. Several number of plants used in traditional medicines for rejuvenation therapy and chronic ailments have been shown to stimulate immune responses and several active substances had been isolated [2]. In clinical medicine both facts of immunomodulation viz. stimulation and suppression are of equal importance. The study of immunomodulation has a role to play in various fields varying from opportunistic infections to medical oncology [3]. Usage of plants as a source of immunomodulators presents a promising future, since they are cost effective, have a broad spectrum activity and may have no side effects [4-5]. Several studies have previously attempted the immunomodulating effects of medicinal plants on lymphocyte proliferation in the presence of mitogen, allogenic cells and specific antigen [6-8]. The present study was undertaken to test the methanol extract of four different medicinal plants Solanum nigrum L. (Solanaceae), Indigofera tinctoria L. (Fabaceae), Euphorbia hirta L. (Euphorbiaceae) and Azima tetracantha L. (Salvadoraceae) for their immunomodulatory activity in vitro.
Materials and Methods
Plant materials
The plant materials as a whole were collected during the month of March 2006 from Kolli hills, Tamil Nadu, India, through interviews and questionnaire among the tribal practitioners for various ailments like skin diseases, ulcer, asthma, cough and hydrophobia. The plant materials were taxonomically identified and voucher specimen deposited in the department herbarium, Loyola College, Chennai (India). Fresh plant materials were washed under running water, shade dried and the parts were coarsely powdered and stored in air tight bottles.
Extraction
Ten grams of dried plant material were extracted with 100 ml of methanol kept on a rotary shaker for 48 h. Thereafter, it was filtered and centrifuged at 5000 g for 15 min. The supernatant was separated and the solvent was evaporated to make the final volume one-fifth of the original volume [9]. The extracts were filter sterilized and stored at 40C until use.
Cell culture
Vero cells (African green monkey kidney cell) were obtained from National Centre for Cell Science, Pune, India, grown in EMEM (Eagle’s minimum essential medium) supplemented with Earle’s salts and 10 % heat inactivated NBCS (New born calf serum), 100 IU/ml penicillin, 100 µg/ml streptomycin and 50 µg/ml gentamycin. The cells were maintained at 370C in a humidified atmosphere with 5 % CO2 and were subcultured twice a week.
Cytotoxicity assay
Each methanol extracts were dissolved separately in 1 ml of 20 % DMSO (Dimethyl sulphoxide), filter sterilized and further diluted to attain concentration of 2 mg/ml, 1 mg/ml, 0.5 mg/ml, 0.25 mg/ml, 0.125 mg/ml and 0.062 mg/ml. 100 µl of cell suspension containing 5×106 cells seeded onto a 96-well microtitre plate. 100 µl of different of concentrations of extracts (2 mg/ml, 1 mg/ml, 0.5 mg/ml, 0.25 mg/ml, 0.125 mg/ml and 0.062 mg/ml) were added after 24 h of seeding. Control consisted of cells without extract, and with DMSO. The microtitre plates were incubated at 370C in a humidified incubator with 5% CO2 for period of 72h. The morphology of the cells were inspected daily and observed for microscopically detectable alterations.
Isolation of Peripheral blood mononuclear cells (PBMC)
PBMC were obtained from healthy adult volunteers by centrifugation of heparinized venous blood over Ficoll/Hypaque solution (Histopaque, Sigma, St. Louis MO). Mononuclear cells were collected from the interphase, washed three times in RPMI-1640. Their viability was determined by trypan blue exclusion test [10]. Cell suspensions were adjusted to 5×106 cell/ml and suspended in RPMI-1640 supplemented with 10% FCS. 100 µl of cell suspension solution was placed in each well of a 96 well flat bottom microtitre plate (Nunc 167008, Nuncton, Roskilde, Denmark). The cells were incubated for 24 h. After incubation, phytohaemagglutinin (PHA) (Gibco BRL, Gaithersberg MD) were added to the control wells (20 µg/ml). The extracts at different concentrations (50, 100, 150µg/ml) were co-cultured with or without PHA. The plates were incubated in 5% CO2 air humidified atmosphere at 370 C for 72 h. Subsequently 3H-Thymidine (50 ci/µmol, 1µci/well, Amersham USA) was added into each well. After 16 h incubation, the cells were harvested on glass fiber filters. Radioactivities in the filters were measured by a scintillation counter (Packard TRI CARB 2100 TR USA) in Counts Per Minute (CPM). Controls consisted of PBMC with PHA (100 % activity) and PBMC with medium (0 % activity)
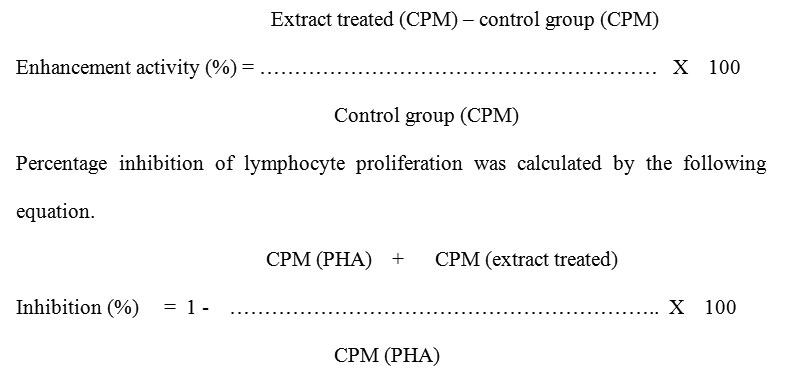
The enhancement and inhibition activity of each extract on lymphocyte proliferation was calculated by the following equation [11].
RT-PCR analysis for expression of IL-4 and IFN- γ
PBMC were isolated, washed twice and resuspended in RPMI – 1640 supplemented with 100µg/ml penicillin, 50 µg/ml gentamycin, 2mM L-glutamine, 10% heat inactivated fetal calf serum (FCS). Non activated PBMC and PBMC activated with PHA (GIBCO BRL, Gaithersberg, MD) at 20 µg/ml were adjusted to a final concentration of 5 x 106 cells/ml. Plant extracts at different concentrations (50, 100, 150µg/ml) were added to the PBMC, cells were cultured in flat bottom 96 well micro titer plates in duplicates and incubated in 5 % CO2 air humidified atmosphere at 370C for 72 hr. RNA were extracted using the guanidium isothio cyanate-phenol chloroform method [12] washed twice in 80% ethanol-diethyl pyrocarbonate (DEPC) treated water and dried. The samples were resuspended in 8 µl – DEPC treated water and reverse transcribed using Moloney Murine Leukemia Virus Reverse Transcriptase (GIBCO BRL, Gaithersburg, MD) and Oligo d(T) priming in a total reaction volume of 20 µl [13]. Amplification of cDNA was carried out using cytokine specific primer pairs with the following 5’ and 3’ sequences [14].
IL-4 5’- ATG GGT CTC ACC TCC CAA CTG CT – 3’
5’- CGA ACA CTT TGA ATA TTT CTC TCT CAT – 3’
IFN-γ 5’ – TGC ATC TTG GCT TTG CAG CTC TCC CTC ATG GC – 3’
5’ – TGG ACC TGT GGG TTG TTG ACC TCA ACC TTG GC – 3’
β- actin 5’ – TGA CGG GGT CAC CCA TGT GCC CAT CTA CTA – 3’
5’ – GAA GCA TTG CGG TGG ACG ATG GAG GG – 3’
5 µl of cDNA was amplified on a thermocycler PTC-100 (MJ Research Inc., Water Town MA). The PCR products (10µl) for IL-4 (656 bp), IFN-γ (456) and β-actin (358 bp) were separated on 1% agarose gel using electrophoresis and visualized by staining with ethidium bromide [15].
Statistical Analysis
All measurements were evaluated statically using student t– test through ANOVA model, taking P <0.05 as significance.
Results
Cytotoxicity assay
All the tested methanol extracts of four different medicinal plants tested for cytotoxicity on Vero cell line did not show any cytotoxicity even at the highest concentration of 2 mg/ml. i.e. loss of monolayer, granulation and vacuolization in the cytoplasm.
Lymphocyte proliferation of 3H thymidine uptake
Stimulation of Lymphocyte proliferation
Out of four different medicinal plant extracts tested for stimulation of lymphocyte proliferation, A. tetracantha (100µg/ml) showed a maximum of 76%, whereas S. nigrum showed 64% followed by E. hirta 47% and I. tinctoria 38%. 100 µg/ml was taken as an ideal concentration, because the activity was high when compared to 50 µg/ml. 150 µg/ml showed similar to 100 µg/ml (Table 1).
Table 1: Effect of stimulation of methanol extract on PBMC.
| No. | Botanical name | Part used | % Stimulation (50 µg/ml) | % Stimulation (100 µg/ml) | % Stimulation (150 µg/ml) |
| 1 | Solanum nigrum | Leaf | 49±2 | 64±3 | 61±3 |
| 2 | Indigofera tinctoria | Whole | 23±4 | 38±1 | 32±2 |
| 3 | Euphorbia hirta | Leaf | 31±3 | 47±2 | 41±5 |
| 4 | Azima tetracantha | Leaf | 58±2 | 76±1 | 70±3 |
% of stimulation of lymphocyte at 50, 100 & 150µg/ml, measured by 3H-thymidine incorporation in DNA, after 72 h incubation in 370C at 5% Co2 atmosphere.
Data are reported as the mean ± S.D in triplicates. P <0.01 when compared to control (Student t- test).
Inhibition of Lymphocyte proliferation
Among four different extracts tested for inhibition on mitogen induced lymphocyte proliferation at three different concentrations, I. tinctoria showed maximum inhibition of lymphocyte proliferation of 70% followed by A. tetracantha 48%, S. nigrum 47% and least in E. hirta 37%. At 100µg/ml, it was maximum inhibition of lymphocyte proliferation when compared to 50 and 150 µg/ml (Table 2).
Table 2: Effect of inhibition of methanol extract on PBMC.
| No. | Botanical name | Part used | % inhibition (50 µg/ml) | % inhibition (100 µg/ml) | % inhibition (150 µg/ml) |
| 1 | Solanum nigrum | Leaf | 28±3 | 47±2 | 42±4 |
| 2 | Indigofera tinctoria | Whole | 60±2 | 70±1 | 68±1 |
| 3 | Euphorbia hirta | Leaf | 21±4 | 37±2 | 36±5 |
| 4 | Azima tetracantha | Leaf | 40±4 | 48±4 | 43±3 |
% of inhibition of lymphocyte at 50, 100 & 150µg/ml, measured by 3H-thymidine incorporation in DNA, after 72 h incubation in 370C at 5% Co2 atmosphere.
Data are reported as the mean ± S.D in triplicates. P <0.01 when compared to control (Student t- test).
RT-PCR analysis for expression of IL-4 and IFN- γ
RT-PCR techniques were used to test for induction of cytokines IL-4 and IFN-γ for the above mentioned plants. A. tetracantha showed both IL-4 and IFN-γ induction (Figure 1) and I. tinctoria showed IL-4 induction and S. nigrum showed IFN-γ induction (Figure 2). E. hirta showed neither IL-4 nor IFN-γ induction.
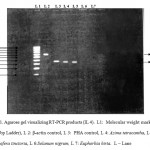 |
Figure 1: RT-PCR for induction of cytokines IL-4.
|
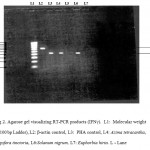 |
Figure 2: RT-PCR for induction of cytokines IFN-γ.
|
Discusion
The present report showed that the methanol extract of leaves from four tested plants, exerts stimulatory and inhibitory potential of lymphocyte proliferation by 3H thymidine incorporation. Similarly methanol extracts from the leaves of Meliaceae were capable of inhibiting the in vitro proliferation of lymphocytes and several immune responses in which these cells are involved [16]. Davis and Kuttan [17] reported Viscum album, Panax ginseng and Tripterygium wilfordi were known to have immunomodulatory activity. A number of facts can influence the lymphocyte proliferation assays – the extraction methods, lymphocyte proliferation, culture medium composition, pH, concentration of mitogen and incubation temperature.
For immunostimulation, A. tetracantha showed higher percentage 76% and lowest in I. tinctoria 38%. Similar results were observed by Wong and Tan [18]. Whereas the extracts of Rhaphidophora korthalsi found to stimulate the lymphocyte proliferation at similar concentration. Its chemical constituents include flavonoids, triterpenoids and alkaloids [19].
tinctoria showed maximum inhibition (70%) on mitogen induced lymphocyte proliferation. Almeida et al., [20] showed that an enriched saturated fatty acid fraction from Kalanchoe pinnata presents inhibitory activity on lymphocyte proliferation and traditionally it has been used as antioxidant and hepatoprotective agent. Before performing the assay trypan blue viability test were performed for 100 % viability of PBMC. After 24, 48 and 72 h all the extract treated cells were checked for viability and in each case their viability percentage was more than 85%. So in the present study we have observed that the inhibitory effects could not be considered as the toxic effect of the plants.
Lymphocyte proliferation activity may also be due to the direct effect of plant extracts or may be mediated through activated release of cytokines such as IL-4, IFN-γ. In several studies increase in cytokine production due to herbal plants has been shown by Haq et al., 1999 [21] and seed extracts of Aeginetia indica induced IL-2, IFN-γ, IL-6 production and lymphocyte proliferation invitro [22]. Immunostimulatory activity of andrographolide is evidenced by increased IL-2 and TNF-2 production and enhancement of lymphocyte proliferation, resulting in strengthened responses and cytotoxic activity of lymphocytes against cancer cells [23, 24]. Among the four plants, IFN-γ induction observed in Azima tetracantha and Solanum nigrum. While Indigofera tinctoria and Euphorbia hirta did not show IFN-γ activity. Plant derived natural products such as flavonoids, terpenoids and steroids etc. have received considerable attention in recent years due to their diverse pharmacological properties [25]. The reported activity of these plants may be due to the presence of secondary metabolites. From the above studies we suggested that immunomodulatory agents present in these plants can enhance the immunological responsiveness by interfering with its regulatory mechanisms. These agents may selectively activate either cell mediated or humoral immunity by stimulating either Th1 or Th2 type of T cell response respectively. Further investigation should be considered in the effects of extracts and its components in interfering with cytokine production by in vivo models.
References
- Ramanathan SK, Thangavel S, Rajagopal SS, Palanavel S, Ramalingam N, Malaya G, Upal KM. Antimicrobial and Antioxidant activities of Careya arborea Roxb. Stem bark. Iranian J of Pharm & Therapeutics. 2006;5: 35-41.
- Mungantiwar AA, Nair AM, Shinde UA, Dikshit VJ, Saraf MN, Thakur VS, Sainis. Studies on the Immunomodulatory effects of Boerhovia diffusa alkaloid fraction. J. Ethnopharmacol. 1999;65: 125-131.
- Bhagwan Dash V. Fundamentals of Ayurvedic medicine. Bansal & Co., Delhi, India; 1978;9-16.
- Atal CK. Role of some important Ayurvedic drugs in modulating the immune system in human body. Indian Drug Manufactures Association Bulletin. 1985;16-17.
- Atlas CK, Sharma ML, Kabul A, Kauri A. Immunomodulatory agents of plant origin, preliminary screening. J. Ethnopharmacol. 1986;18:133-141.
- Lamm DL, Riggs DR. The potential application of Allium sativum (garlic) for the treatment of bladder cancer. Urol Clin North Am. 2000;27:157-62.
- Summerfield A, Saalmuller A. Interleukin-2 dependent selective activation of porcine gamma delta T lymphocyte by an extract from the leaves of Acanthospermum hispidum. Int J Immuno pharmacol. 1998;20(1-3):85-98.
- Akesson C, Pero RW, Ivass F. C-Med 100, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo. Phytomed. 2003;10:23-33.
- Parekh J, Nair R, Chanda S. Preliminary screening of some folkloric plants from western India for potential antimicrobial activity. Indian J Pharm. 2005;37:408-409.
- Moldeus P, Hogberg J, Orrenius S, Fleischer S, Packer L. Isolation and use of liver cells. Methods in enzymology, New York: Academic Press: 1978;52:60-68.
- Lie-Chwen L, Yuh-Chi K, Cheng-Jen C. Immunomodulatory principles of Dichrocephala bicolor. J. Natural products. 1999;62(3):405-408.
- Chomczynski P, Sacchi N . Single step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal. Biochem. 1987;162(1):156-159.
- Ehlers S, Smith KA. Differentiation of T cell lymphokine gene expression: The in vitro acquisition of T cell memory. J. Exp. Med. 1991;173:25-36.
- Mullis KB. The unusual origin of polymerase chain reaction. Sci. Am. 1990;262: 56-65.
- Innis MA, Gelfend D, Sninsky JJ, White TJ. Eds. PCR protocols: A guide to Methods and Applications. New York: Academic press. 1990;3-12.
- Benecia F and Coulombie FC. Immunomodulatory activities of Trichila glabra leaf aqueous extracts. Phytother. Res. 1998;12(3):167-171
- Davis L, Kuttan G. Immunomodulatory activity of Withania somnifera. J. Ethno pharmacol. 2000;71:193-2000.
- Wong KT, and Tan BKH. In vitro cytotoxicity and immunomodulating property of Rhaphidophora korthalsi. J. Ethnopharmacol. 1996;52(5): 53-57.
- Bennett RN, Mellon FA, Rosa EA, Perkins L and Kroon PA Profiling glucosinolates, flavonoids, alkaloids, and other secondary metabolites in tissues of Azima tetracantha L. (Salvadoraceae), Journal of Agricultural and Food Chemistry 2004;22:5856–5862.
- Almeida AP, Da-silva SAG, Souza MLM, Lima LMTR, Rossi Bergmann B, Goncalves de Moraes VL. Isolation and chemical analysis of a fatty acid fraction of Kalanchoe punnata with a potent lymphocyte suppressive activity. Planta Med. 2000; 66: 134-137.
- Haq A, Lobo PI, Al-Tufail M, Rama NR, Alsedairy ST . Immunomodulatory effect of Nigella sativa proteins fractionated by ion exchange chromatography. Int.J. Immunopharmacol. 1999;21(4):283-295.
- Chai JG, Bando T, Nagasawa H, Himeno K, Sato M, Oh kubo S. Seed extract of Aeginetia indica L. induces cytokine production and lymphocyte proliferation in vitro. Immunopharma. 1994;27(1): 13-21.
- Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004;92: 291-295.
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide a potential cancer therapeutic agent isolated from Andrographis paniculata. J.Exp.Ther. oncol. 2003;3:147-158.
- Bhandary MJ, Chandrash Sekhar KR, Kaveriappa KM. Medical ethnobotany of the Siddis of Uttara Kannada District, Karnataka, India. J Ethnopharmacol. 1995;47(3):149-58.

This work is licensed under a Creative Commons Attribution 4.0 International License.





