How to Cite | Publication History | PlumX Article Matrix
An Efficient Protocol for Micro Propagation of Gardenia Jasminoides Ellis.
Siham Abd Al-Razzaq Salim and Sumaya Younus Hamza
Al-Musaib Technical College, Department of Plant Production Techniques, Iraq.
Corresponding Author E-mail: dr.sihamabdalrazzaq@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2505
ABSTRACT: The purpose of present study was the development of a suitable protocol for micro propagation of Gardenia jasminoides by using explants from shoot tips and nodes exist from 2-3 years old plants. The sterile explants cultured in MS medium supplemented with TDZ ( 0.0, 1.0, 2.0, 3.0 or 4.0 mg/L ) in combination with IAA ( 0.0, 0.1, 0.2, 0.3 or 0.4 mg/L) for shoot initiation. Results showed there was no significant difference between explants source in initiation stage. The highest averages of shoots numbers, shoots lengths, number of leaves and nodes per shoot were obtained at combination 3.0 mg/L TDZ + 0.3 mg/L IAA for both explants. The micro shoots from initiation stage were harvested and cut into new explants and transferred to multiplication medium containing the same concentrations of TDZ and IAA above in addition of 3.0 mg/L of GA3 of all treatments. The best results of shoots multiplication through shoots numbers(3.8 shoots/explant), shoots lengths(3.2 cm), number of leaves(6.9 leaves/shoot) and nodes(5.2 nodes/shoot) were obtained at 3.0 mg/L TDZ + 0.3 mg/L IAA. Also, there was induction of flower buds formation in some TDZ and IAA combinations. Then, the multiplied shoots were transferred to rooting medium comprised of half strength of MS salts containing 4.0 g/L activated charcoal and supplemented with IBA ( 0.0, 0.5, 1.0 or 1.5 mg/L) and NAA ( 0.0, 0.5, 1.0, 1.5 or 2.0 mg/L ) individually. Results showed the concentration 1.5 mg/L of IBA gave the highest rooting response(90%), whereas, the concentration 1.0 mg/L IBA gave highest average of roots number (3.1 roots/shoot) and roots length(4.6 cm). While, the concentration 1.0 mg/L of NAA gave the best rooting response (80%), roots number (2.4 roots/shoot) and roots length(2.37 cm) after 6 weeks of culture in rooting medium. Rooted plants were transplanted to sterile mixture of peat moss and river soil (2:1) in pots for acclimatization, and after 4 weeks, the plants became hardened and gave survival percentage of 86% of plants.
KEYWORDS: Gardenia jasminoides; micropropagation; ornamental plants
Download this article as:| Copy the following to cite this article: Salim S. A. A, Hamza S. Y. An Efficient Protocol for Micro Propagation of Gardenia Jasminoides Ellis. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Salim S. A. A, Hamza S. Y. An Efficient Protocol for Micro Propagation of Gardenia Jasminoides Ellis. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=26163 |
Introduction
Gardenia jasminoides Ellis, is an ornamental and medicinal woody plant belonging to the family Rubiaceae. The genus name Gardenia was given to commemorate Dr. Alexander Garden ( 1780-1791 ), and there are over than 200 species of this genus. Gardenia plants are evergreen shrubs with dark green glossy and oval leaves. Flowers are white with sweet fragrance. It is a popular plant used as a cut flower, screens, hedges, borders or ground covers. This genus is native in tropical and subtropical regions of Asia, Africa, Australia and Oceania.1,2 Cultivars of G. jasminoides can be propagated conventionally by cuttings or grafting. Terminal cuttings result in low proliferation rates, and the grafting can be doing by a scion from a desired cultivar grafted on a seedling rootstock of G. thunbergia ; which is grown primarily as rootstock. However , the rootstock seedlings are difficult to obtain due to the seed germination problems. Micro propagation of ornamental, aromatic and medicinal plants has been achieved by shoot tips , axillary buds and single nodes through plant tissue culture technique offers high rates of shoots proliferation per each starting plant.3,4,5
Cytokinins and auxins were used as single or in combination for inducing cell divisions, as well as for the proliferation of shoots of many plants.6 used young shoots as explants for shoot formation of G. jasminoides in MS medium supplemented with BA at 10 mg/L or 7.5 mg/L 2iP. In the study of,7 they used shoot tips and single nodes of Rosa sp. As explants and cultured them on MS medium containing different concentrations of BA , the found that 1.5 mg/L BA gave the highest rate of shoot multiplication 8 shoots/explant in single nodes than shoot tips ( about 5 shoots/explant).8 stimulated shoot proliferation from in vitro culture of single nodes of G. jasminoides using MS medium supplemented with Kin and NAA. In the study of,9 they induced axillary shoot formation in Cassia angustifolia using different concentrations of TDZ. While10 found that the culture medium containing 0.1 mg/L BA with 0.2 mg/L NAA produced maximum shoot number of Eustoma grandiflorum . On the other hand, MS medium supplemented with 3.0 mg/L BAP with 0.1 mg/L NAA was the best for shoot regeneration and multiplication in Gerbera jamesonii .11
In the study of,12 they found the best shoot rooting of G. jasminoides was achieved in half strength MS medium supplemented with different concentrations of IAA.13 reported in their study the highest percentage of shoot rooting was 91.66% and roots length ( 4.62 cm) when used IBA at 1.0 mg/L . Whereas14 used IAA, IBA and NAA at concentrations of 0.0, 0.1, 0.5 or 1.0 mg/L for rooting of in vitro propagated shoots of Chrysanthemum morifolium , and found that 1.0 mg/L of IBA was the best for rooting frequency, roots number per explant and roots length among the three different concentrations of auxins used , followed by IAA and NAA. The aim of this study was to evaluate the using of different explants and type and concentrations of plant growth regulators on micro propagation of Gardenia jasminoides.
Materials and Methods
This study was carried out at the Plant Tissue Culture Laboratory , Department of Plant Production Techniques, Al-Musaib Technical College.
Preparation and Sterilization of Explant
Young and health shoots with leaves of G. jasminoides, about 8 cm in length were collected from 2-3 years old parental plants. In the laboratory and after removing leaves , the shoots were divided into shoot tips and nodal segments to be used as explants, and they washed with tap water and liquid soap, and left under running tap water for 30 minutes. Then , they were soaked in cold antioxidant solution containing ascorbic acid (150 mg/L) and citric acid ( 100 mg/L) in the ratio 1:1 for 30 min. to decrease the browning of tissues, followed by rinsing in sterilized distilled water. For surface sterilization, the selected explants immersed in a freshly prepared aqueous solution of mercuric chloride(HgCl2) at concentration 0.1%(w/v) for 5 min. Finally, they were rinsed with sterile distilled water for 5 min. each for 3 min. to remove any traces of sterile solution. After that, explants were cut into shorter sections( shoot tips to 0.5-1.0 cm in length and nodal segments to 1.5 cm in length) to be cultured.
Preparation of MS Medium
Murashige and Skoog ( MS ) powder medium15 was used in the present study. MS powder ( 4.43 g) and 30 g of sucrose were weighed out and were added into 1000 ml flask filled with 800 ml of distilled water on a magnetic stirrer to dissolve the powders, then , the volume was adjusted to 1000 ml with distilled water. After the addition of appropriate concentrations of plant growth regulators, the pH of medium was adjusted to 5.7 using few drops of 0.1-1.0 N of NaOH or HCl. Then 7.0 g of agar was added and the medium was heated on the hot plate magnetic stirrer until agar was completely dissolved. Finally , the medium was distributed into screw-cap containers and was sterilized in autoclave at 121 ºC under a pressure of 1.04 Kg/cm2 for 15 minutes.
Shoot Initiation Stage
Selected explant ( shoot tips and nodes ) were cultured on MS medium supplemented with different concentrations of the cytokinin TDZ ( 1.0, 2.0, 3.0 or 4.0 mg/L ) alone or in combination with the auxin IAA ( 0.1, 0.2, 0.3 or 0.4 mg /L ), as well as the control treatment ( free-hormone medium). Ten replicates for each concentration were cultured. All cultures were incubated in the culture room at temperature 25 ± 2 ºC under 16 hours photoperiod using cool fluorescent tubes of light intensity of 1000 lux. Results were taken after 8 weeks of culture through the following : numbers and lengths of proliferated shoots, number of leaves and nodes per shoot, chlorophyll content , fresh and dry weights of shoots.
Shoot Multiplication Stage
Micro shoots that produced from initiation stage were cut and transferred to MS medium containing TDZ and IAA at the same concentrations that mentioned above. Gibberellic acid ( GA3 ) was added at concentration 3.0 mg/L to all treatments for shoot elongation at this stage. Ten replicates were cultured for each concentration, and cultures were incubated at the same growth conditions above for 8 weeks. Results were recorded via the following parameters: number and lengths of multiplied shoots , number of leaves and nodes of leaves per shoot, chlorophyll content , fresh and dry weights of shoots.
Rooting Stage
After the multiplied shoots were reached to 2-3 cm in length, they were transferred into half strength MS medium supplemented with different concentrations of IBA ( 0.0, 0.5, 1.0 or 1.5 mg/L ) or NAA ( 0.0, 0.5, 1.0, 1.5 or 2.0 mg/L ) individually. Activated charcoal was added to all media at 4.0 mg/L. Ten replicates were cultured for each concentration. Cultures were incubated in culture room under the same growth conditions that mentioned above. Results were recorded after 6 weeks through: percentage of rooting, number and length of roots.
Acclimatization Stage
After the plantlets were rooted, they were taken out from rooting medium and thoroughly washed with running tap water to remove any traces of agar. They were treated with fungicide solution ( Benlate 0.1 % w/v) and they transferred to pots containing sterilized mixture of peat moss and river soil in the ratio 2:1 . Pots were covered with transparent covers that were removed from time to time after two weeks of culture, and after 4 weeks, covers were completely removed. The percentage of survival rate of acclimatized plantlets was calculated.
Statistical Analysis
Results were analyzed using completely randomized design ( CRD ) , and the means were compared using the least significant difference ( LSD ) test at property of 0.05.16
Results and Discussion
Initiation Stage
Results in table ( 1 ) and figure ( 1 ) showed the effect of TDZ , IAA and explant source on the average number of proliferated shoots, length of shoots, average number of leaves and nodes per shoot. It is clear that there is no significant difference between shoot tip and nodal explant on the studied features. The using of TDZ at different concentrations was more effective in obtaining highest averages of shoots number, shoots length, leaves number and nodes number in both shoot tip and nodal explants( 1.88, 1.76 shoots/explant, 1.32, 1.95 cm, 3.18, 3.10 leaves/shoot and 1.88, 2.06 nodes/shoot respectively). This result was in agreement with6 and17 that obtained high proliferation of shoots of G. jasminoides through using other types of cytokinins: BA, 2iP and Kin, but no reports had been recorded about TDZ in shoot induction from shoot tip or node in G. jasminoides . Thidiazuron which is known as TDZ is one of the cytokinins that used for stimulation cell division , as well as for the formation and growth of shoots and more effective with woody and ornamental plants.18 As regards to IAA concentrations , the concentration 0.3 mg/L was significantly overcame on other IAA concentrations in shoots number , shoots length , number of leaves and nodes per shoot for both shoot tip and nodal explants ( 2.1, 2.2 shoots/explant, 1.3, 1.1 cm, 3.6, 3.1 leaves/shoot and 2.4, 2.3 nodes/ shoot respectively ).
According to the interaction among TDZ, IAA and explant source, it is clear that the highest averages of shoots number, shoots length, number of leaves and nodes per shoot were obtained at the interactions: 3.0 mg/L TDZ + 0.3 mg/L IAA for both explants ( 3.3, 4.2 shoots/explant , 1.70, 1.95 cm, 5.4, 5.8 leaves/shoot and 3.3, 4.2 nodes/ shoot respectively ) as compared with control treatment. Most of explants that were used in tissue culture can proliferate shoots without addition of any growth regulators depending on their endogenous hormones that may be not sufficient to produce shoots, but the induction of more shoots can be increased with the incorporation of plant growth regulators specially cytokinins and auxins in this stage which is very important to grow and form vegetative shoots.19,20,10
Results in table ( 2 ) referred to the effect of TDZ, IAA and their interactions on chlorophyll content , fresh and dry weights of proliferated shoots. The concentration 3.0 mg/L of TDZ was significant for chlorophyll content ( 3.54 µg/mg ), but not significant for fresh and dry weights of shoots. Also, IAA at 0.3 mg/L was the best for chlorophyll content ( 3.86 µg/mg ) , but not significant for fresh and dry weights. Concerning the interaction between TDZ and IAA, the medium supplemented with 3.0 mg/L TDZ + 0.2 mg/L IAA gave the highest and significant average of chlorophyll content ( 6.17 µg/mg ) as compared with control, whereas , MS supplemented with 3.0 mg/L TDZ + 0.1 mg/L IAA gave highest fresh weight ( 488 mg ) and 2.0 mg/L TDZ + 0.3 mg/L IAA was the best for shoot dry weight ( 86.7 mg ). Generally , the effect of plant growth regulators specially cytokinins in their role in activate the chlorophyllase enzyme which active chlorophyll synthesis and their role in activate the absorption of water by growing shoots and translocation of nutrients in shoot , also , the presence of sucrose in the medium resulted in the carbohydrates accumulation in growing shoots which encouraged by growth regulators lead to the increasing of fresh and dry weights of shoots.21,22
 |
Table 1: Effect of explant source, TDZ, IAA and their interactions on average number of proliferated shoots, length of shoots, average number of leaves and nodes of proliferated shoots in initiation stage.
|
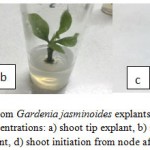 |
Figure 1: Initiation of shoots from Gardenia jasminoides explants on MS medium supplemented with TDZ + IAA at different concentrations: a) shoot tip explant, b) shoot initiation from shoot tip after 8 weeks, c) node explant, d) shoot initiation from node after 8 weeks of culture.
|
 |
Table 2: Effect of TDZ, IAA and their interactions on average number of chlorophyll content, fresh and dry weights of proliferated shoots in initiation stage.
|
Multiplication Stage
Table ( 3 ) and figure ( 2 ) showed the effect of different concentrations of TDZ , IAA and their interactions on the average number of multiplied shoots , length of shoots, number of leaves and nodes per multiplied shoots. There was a significant increasing in shoot numbers, shoot length, number of leaves and nodes per shoot with the increasing of TDZ concentrations to reach the highest averages at 3.0 mg/L TDZ ( 2.42 shoots/explant, 2.02 cm, 4.3 leaves/ shoot and 3.5 nodes/shoot ). The needing of addition of cytokinins for shoot multiplication medium is recorded by previous studies.23,8,10 Using of the concentration 0.3 mg/L of IAA gave the highest averages of shoot number ( 2.12 shoots/explant ), shoot length ( 1.66 cm ), number of leaves ( 3.02 leaves/ shoot ) and number of nodes ( 2.64 nodes/ shoot ). Concerning the interaction between TDZ and IAA, the medium supplemented with 3.0 mg/L TDZ + 0.3 mg/L IAA gave the highest averages in shoot number (3.8 shoot/explant), highest length ( 3.2 cm ), highest number of leaves (6.9 leaves/shoot) and highest number of nodes (5.2 nodes/ shoot). The present study revealed the induction of flower buds in some TDZ and IAA interactions.
The multiplication stage is an important in micro propagation of plants to get faster growth rates . furthermore, many previous studies referred to the importance of interaction between cytokinins and auxins in shoot multiplication due to the dual effective of these regulators on the cell division and cell enlargement, so a larger number of buds will have to grow and start to produce new shoots [7]. Also, in the presence of GA3 in multiplication medium was important for increasing shoot length, so that many studies used GA3 with cytokinins and auxins in culture medium to help in breakdown dormancy and to increase shoot length in initiation and multiplication stages [24] [25]. This may explained the induction of flower buds formation.
As shown in table ( 4 ), the results displayed the effect of TDZ, IAA and their interaction on chlorophyll content, fresh and dry weights of multiplied shoots , TDZ at 3.0 mg/L increased chlorophyll content ( 3.54 µg/mg) and dry weight ( 106.2 mg ) of multiplied shoots, but not significant in fresh weight as compared with other concentrations. On the other hand, using of IAA at 0.2 mg/L was best for chlorophyll content, whereas IAA concentrations 0.1 and 0.3 mg/L did not differ significantly from control in fresh weight, while the highest average of dry weight ( 101.6 mg ) was obtained at 0.3 mg/L IAA. Regarding to the TDZ and IAA interaction, it is clear that the interaction 3.0 mg/L TDZ + 0.2 mg/L IAA was the best for chlorophyll content ( 6.17 µg/mg ), while the highest average of fresh weight ( 843 mg ) was obtained at 3.0 mg/L TDZ + 0.0 mg/L IAA. Also, the interaction 3.0 mg/L TDZ + 0.1 mg/L IAA gave the highest average of shoot dry weight ( 150 mg ). The interaction between cytokinins and auxins is very important in vegetative multiplication can be interpreted by the increasing of cytokinine role in the presence of auxins resulting to activate chlorophyll synthesis, absorption of water and increasing the carbohydrates rates accumulation which resulted in fresh and dry matter of multiplied shoots [21] [22] .
 |
Table 3: Effect of TDZ, IAA and their interactions on average number of multiplied shoots, length of shoots , average number of leaves and nodes of multiplied shoots in multiplication stage. ( all media containing 3.0 mg/L GA3).
|
 |
Figure 2: Multiplication and elongation of Gardenia jasminoides shoots on MS medium supplemented with TDZ+IAA at different concentrations with 3.0 mg/L GA3 after 8 weeks of culture: a) shoot multiplication, b) and c) different lengths of elongated shoots, d) in vitro formation of flower bud ( arrow).
|
 |
Table 4: Effect of TDZ, IAA and their interactions on average number of chlorophyll content, fresh and dry weights of multiplied shoots in multiplication stage ( all media contain 3.0 mg/L GA3).
|
Rooting Stage
Results in table ( 5 ) and figure ( 3 ) showed the effect of IBA and NAA concentrations individually on rooting response, average number of roots and roots length of G. jasminoides micro shoots that were cultured on half strength MS medium ( containing 4.0 g/L activated charcoal ) after 6 weeks of culture. It can be noticed that there was no significant difference among IBA concentrations in rooting response from each other, but were significant as compared with control treatment ( MS free hormone medium which did not respond to root ), whereas the highest averages of roots number and roots length were obtained at 1.0 mg/L IBA ( 3.10 roots/shoot and 4.60 cm respectively ). Results from same table, showed that the concentration 1.0 mg/L NAA gave the highest rooting response ( 80 % ), highest average of roots number ( 2.4 roots/shoot ) and highest length of roots ( 2.37 cm ).
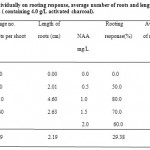 |
Table 5: Effect of IBA and NAA individually on rooting response, average number of roots and length of roots of micro shoots cultured in half strength MS medium ( containing 4.0 g/L activated charcoal).
|
These results were in agreement with those observed by [13] [26] [11] , who found that the reduction of MS salts levels in medium to half strength increased rooting of many plant species. The decreasing in MS medium salts levels to half or quarter means the decreasing in nitrogen levels in medium and then in shoots which causing the increasing in carbohydrates accumulation and this may result in increasing the root primordial and root numbers [27]. Furthermore, the results proved that the auxins have an important role in rooting process, since they promote adventitious roots initiation in the bases of in vitro cultured shoots, so the exogenous supplying of auxins at optimal concentration would increase this process [28].
Acclimatization Stage
The rooted plantlets were remove carefully from the rooting medium and transplanted in plastic pots containing sterile mixture of peat moss and river soil ( 2:1) and covered with plastic and transparent covers and gradually removing the covers after 2 weeks to harden plantlets. After 4 weeks, the plantlets were acclimatized and the survival percentage reached to 86% of plantlets ( figure- 4 ). The acclimatization of micro propagated plants was important to help the plantlets to be converted from heterotrophic to autotrophic by promoting photosynthetic enzymes.29 Following these steps agree with the findings of researchers of various ornamental plants.30,10,14
 |
Figure 3: Root initiation of Gardenia jasminoides micro shoots on half strength MS medium containing 4.0 mg/L activated charcoal and supplemented with different concentrations of : a) IBA ; from left to right : 1.5 , 1.0, and 0.5 mg/L. b) longest root length ( 11 cm at 1.0 mg/L IBA ). c) NAA ; from left to right : 2.0, 1.5, 1.0 and 0.5 mg/L after 6 weeks of culture.
|
 |
Figure 4 a: acclimatization of Gardenia jasminoides microplants in pots. b) hardened plant after 3 months of acclimatization ready to transfer to green house.
|
Conclusion
According to findings of present study, the micro propagation of Gardenia jasminoides using different explants and plant growth regulators is very successful for in vitro propagation of this plant in the future.
References
- Green P. S. The Name Jasmine. A Continuation of The Bulletin of Popular Information of The Arboretum, Harvard University. Arnolda, USA: Harvard University. 1965.
- Wilkins H. F. Gardenia jasminoides . In: Handbook of Flowering. Edited by Halevy AH. 1986;5:127-131.
- Razdan M. K. Introduction to Plant Tissue Culture. 2nd Ed., Science Publishers, Inc. 2003.
- George E. F.,Hall M. A and DeKlerk G. J. Plant Propagation by Tissue Culture, Third Edition, Springer, Dordrecht, Netherlands. 2008.
- Chavan J. J.,Gaikwad N. B., Umdale S. D.,Kshirsagar P. R.,Bhat K. V and Yadav S. R. Efficiency of direct and indirect shoot organogenesis, molecular profiling, secondary metabolite production and antioxidant activity of micro propagated Ceropegia santapaui . Plant Growth Reg. 2014;72:1-15.
CrossRef. - Chuenboonngarm N.,Charoonsote S,and Bhamarapravati S. Effect of BA and 2iP on shoot proliferation and somaclonal variation of Gardenia jasminoides Ellis in vitro culture. Sci. Asia. 2001;27:137-141.
CrossRef. - Roy P. K.,Mamun A. N. K and Ahmad G. In vitro plantlets regeneration of Rose. Plant Tiss. Cultu. 2004;14(2):149-154.
- Duhoky M. M. S and Rasheed K. A. Effect of different concentrations of Kinetin and NAA on micropropagation of Gardenia jasminoides. J. Zankoy Sulai. 2010;13:103-120.
CrossRef - Parveen S and Shahzad A. A micro propagation protocol for Cassia angustifolia Vahl. from root explants. Acta Physiol. Plant. 2011;33:789-796.
CrossRef. - Kaviani B., Zamiraee F.,Zanjani S. B.,Tarang A and Torkashvand A. M. In vitro flowering and micropropagation of lisianthus( Eustoma grandiflorum ) in response to plant growth regulators( NAA and BA). Acta Sci. Pol. Hort. Cultu. 2014;13(4):145-155.
- Shylaja M. R.,Sashna P.,Chinjusha V and Nazeem P. A. An efficient micro propagation protocol for Gerbera jamesonii Bolus from flower buds. Inter. J. Plant Anim. Environ. Sci. 2014;4(3):641-641.
- Abdullah G. R.,Al-Khateeb A. A and Serage M. Effect of different concentrations of growth regulators on Gardenia jasminoides cv. Veitchii micro propagated by tissue culture technique. J. Agric. Marine Sci. 2003;8(1):35-40.
CrossRef. - Salahaddin M.,Nasirujjaman K., Maman S and Reza M. A. Regeneration of multiple shoots from different explants viz. shoot tip, nodal segment and cotyledonary node of in vitro growing seedlings of Peltophorum pterocarpum (D.C.) Backer ex K. Heyne. Biotechnology. 2005;4(1):35-38.
CrossRef. - Chae S. C. Influence of auxin concentration on in vitro rooting of Chrysanthemum morifolium Ramat. Bio. Sci. Biotech. Res. Asia. 2016;13(2):833-837.
CrossRef. - Murashige T and Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962;15:473-497.
CrossRef. - GenStat . Gen Stat Procedure Library Release PL 18.2. Edition 4. VSN International Ltd. Roth Amsted Experimental Station. UK. 2012.
- Sayd S. S.,Taie H. A and Taha S. L. Micro propagation, an antioxidant activity, total phenolics and flavonoids content of Gardenia jasminoides Ellis as affected by growth regulators. Inter. J. Acad. Res. 2010;2(3):184-191.
- Von A. S. Somatic Embryogenesis. In George E. F.,Hall M. A and DeKlerk G. J. Plant Propagation by Tissue Culture. 2007;342-349. Dordrecht, The Netherlands. Springer.
- Jaykrishna C.,Karthik C.,Barathi S.,Kamalanathan D and ArulSelvi I. P. In vitro propagation of Aloe barbadensis Miller, a miracle herb. Resea. Plant Bio. 2011;1(5):22-26.
- Dereje-Aklile B. In Vitro Propagation of Pepper ( Capsicum annum L.) Through Shoot Tip Culture. MSc. Thesis , Haramaya University, Haramaya, Ethiopia. 2012.
- Tan N. D.,Thi D. H. N.,Trinh D. N., Tanh N. H., Thien N. O and Hong V. N. Effect of genotype, explant size, position and culture medium on shoot generation of Gerbera jamesonii. Sci. Horticultu. 2007;111:146-151.
CrossRef. - Dabrowski P.,Center M. D.,Samborska I. A and Kalaji M. H. Measuring light spectrum as a main indicator of artificial sources quality. J. Coa. Life Medici. 2015;3(5):398-404.
- Murkute A. A.,Patil S and Singh S. K. In vitro regeneration in pomegranate cv. Ganesh from mature trees. Indian J. Hort. 2004;61(3):206-208.
- Shahzad A.,Faisal M and Anis M. Micro propagation through excised root culture of Clitoria ternatea and comparison between in vitro regenerated plants and seedlings. Ann. Appl. Bio. 2007;150:341-349.
CrossRef - Chinnu J. K.,Mokashi A. N.,Hegde R. V.,Patil V. S and Koti R. V. In vitro shoot multiplication and ex vitro rooting of cordyline ( Cordyline sp. ). Karnataka J. Agric. Sci. 2012;25(2):221-223.
- Ozel C. A and Arslan O. Efficient micro propagation of English shrub Rose ” Heritage” under in vitro condition. Inter. J. Agric. Bio. 2006;5:626-629.
- Gawel N. J.,Robacker C. D and Corly W. L. In vitro propagation of Miscanthus sinensis . HortScience. 1990;25(10):1291-1203.
- Hartmann H. T.,Kester D. E.,Davis F. T and Geneve R. L. Plant Propagation: Principles and Practices. 6th Ed., Prentice-Hall, New Jersey. 1997.
- Hazarika B. N. Acclimatization of tissue cultured plants. Curr. Sci. 2003;85:1705-1712.
- Elsheik A. M.,Dafflla H. M and Khalfala M. M. In vitro micro propagation of the ornamental plant Dieffenbachia– A review. Univ. J. Plant. Sci. 2013;1(3):91-99.

This work is licensed under a Creative Commons Attribution 4.0 International License.





