How to Cite | Publication History | PlumX Article Matrix
Department of Environmental Science and Technology, Central University of Punjab, Bathinda (India) – 151401.
DOI : http://dx.doi.org/10.13005/bbra/2921
ABSTRACT:
The current study focused on biotic degradation of waste polyethylene bags using bacterial community from hydrocarbon contaminated soil near coal fired thermal power plant and also the effect of UV irradiation on its biodegradation.The predominant groups in the bacterial community in the hydrocarbon contaminated soil near coal fired thermal power plant were identified by 16s DNA sequencing were Steroidobacter, Flavisolibacter, Planctomyces, Balneimonas, Gemmata, Alicyclobacillus, Lactobacillus, Mycobacterium, Geodermatophilus, Prevotella, Virgisporangium and Adhaeribacter. The native bacterial community from hydrocarbon contaminated soil was capable of polyethylene degradation.The bacterial community in the hydrocarbon contaminated soil metabolized 12.85± 0.16 percent of polyethylene (10 g/L) as sole carbon source in mineral salt media within 30 days.The UV irradiation of polyethylene enhanced weight loss of 22.80 percent higher than untreated polyethylene. The improvement in bacterial degradation by UV exposure of waste polyethylene in-vitro for 144 hresulted 15.78± 0.32 percent weight loss in 30 days. The photo-oxidation by UV irradiation of polyethylene had surface disruption and was confirmed by Field Emission Scanning Electron Microscopy (FE-SEM) and Fourier Transform Infrared Spectroscopy (FTIR). The photochemical reaction induced by UV irradiation of polyethylene resulted in formation of carbonyl peaks on polymer surface and addition as well as shifting of peaks. The morphological changes of polyethylene by UV exposure enhanced colonization, metabolism by and synergistic effect on polyethylene biodegradation by bacterial community from hydrocarbon contaminated soil.
KEYWORDS: Bacterial Community; Carbonyl Index; Waste Polyethylene Bags; Weight Loss,Synergism; UV-Irradiation
Download this article as:| Copy the following to cite this article: Kalia A, Dhanya M. S. Biodegradation of Ultra-violet Irradiated Waste Polyethylene Bags by Bacterial Community from Soil around Coal -fired Thermal Power Plant. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Kalia A, Dhanya M. S. Biodegradation of Ultra-violet Irradiated Waste Polyethylene Bags by Bacterial Community from Soil around Coal -fired Thermal Power Plant. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/3fT9FGJ |
Introduction
Polyethylene is one of the polymers with versatile applications in packaging, insulation, agricultural mulch films,domestic and industrial uses. The polyethylene is categorized based on branching into low-density polyethylene, high-density polyethylene, linear low-density polyethylene and cross-linked polyethylene1. The low-density polyethylene (LDPE) is the prominent component of plastic waste which accounts for 60% of the total plastic production 2. About 94 percent of plastic waste is thermo set plastic and of great concern to the environment3.
The conventional methods of disposal of polyethylene waste include recycling, incineration and landfilling. The biodegradation of polyethylene waste is environment friendly and sustainable than the conventional physicochemical breakdown 4. The biodegradation has been affected by inert and persistent nature of polyethylene for longer time and interference ofhydrophobic nature in polymer availability to micro organisms 5. The microbes with high polyethylene degradation potential were isolated from plastic contaminated sites such as garbage soil, crude oil spilled sites, plastic dumping site and soil form volcano crater6, 7.
The most important step in microbial degradation of polyethylene is the surface attachment of bacterial cells and biofilm formation on the polymer surface8. The alterations in polyethylene properties makeeasy availabilityof polyethylene for microbial biodegradation. The researchers have studied the additive effect of prior abiotic pretreatments of the polymer such as thermal oxidation, UV irradiation and chemical disintegration on microbial biodegradation of polyethylene9.The UV treatment of polyethylene resulted from photo-oxidative reactions by the absorption of ultraviolet radiation10. The enzymes secreted by the microbes initiate the biological degradation of petro-based polymers and break the polymer chain into oligomers and to monomers. These smaller monomeric products are metabolized in the microbial cells as carbon source11.In the present study, the bacterial communityfrom hydrocarbon contaminated soil around coal fired thermal power plant was evaluated for their ability to degrade polyethylene in liquid media in-vitro and also the effect of UV-radiation on its biodegradation.
Material and methods
Collection of Waste polyethylene bags
The waste polyethylene bags werecollected from the local waste dumping site in Bathinda, Punjab, India. The plastic bags were translucent and of 20 microns thickness.The plastic bags were thoroughly washed withdeionized water followed by ethanol sterilization (70% v/v),drying overnight and stored aseptically for further use.
UV irradiation of polyethylene bags
The plastic bags were cut into small strips of 1.5× 1.5 cm, sterilized with 70% v/v ethanol for 30 minutes. The polyethylene strips were irradiated under UV lamps (16W) placing at 5 cm distance for 144h in UV protected glass chamber.
Soil sample forpolyethylene degrading bacterial community
Soil was collected from coal fired Thermal Power Plant, Bathinda, Punjab (30˚13’ 59.57” N and 74˚55’ 48.92” E) from the depth of approximately 0–10 cm in sterile ziplock plastic bags.The bacterial communityused in the present study was prepared by soil enrichment method using waste polyethylene as sole carbon sourcein Mineral Salt media (MSM)12.The bacterial communitywith mixed culture of bacteria was preserved at 4°C and used in further degradation studies.The dominant bacterial groups in the hydrocarbon contaminated soil were identified by16s rDNA sequencing.
Biodegradation of waste polyethylene by bacterial populations
The degradation study was performed with untreated and UV irradiated waste polyethylene (1% w/v) added to mineral salt media as a sole source of carbon and energy at 30°C and continuous agitation of 150 rpm for an incubation period of 1 month.The16 h old bacterial populations weregrown in Luria-Bertani broth was used as the inoculum for degradation studies. The bacterial growth and biodegradation study was investigated by culturing polyethylene enriched medium containingNH4 NO3 (1.0gL-1); K2HPO4(1.0 gL-1); KCl(0·15 gL-1); MgSO4·7H2O (0.2 gL-1); CaCl22H2O (0·1 gL-1) and yeast extract (0·1 (gL-1) along with 1·0 mgL−1 of ZnSO4 7H2O, FeSO4 6H2O, and MnSO413.The samples were collected at 5 days intervals for estimation of biomass growth of bacterial consortium.The weight loss of polyethylene samples from the culture media was estimated14 after washing with sodium dodecyl sulfate (2% (v/v) followed by distilled water15. All the experiments were conducted in triplicates and the results were the mean values with standard deviations. The results were statistically analyzed by single factor Analysis of Variance followed by Post- hoc Tukey test.
Quantification of bacterial adherenceon the polyethylene
The bacterial populations on the polyethylene surface were measured indirectly by determining the concentration of extractable protein. The protein estimation by Lowry’s method was followed for the supernatant obtained from boiling polyethylene samples in 0.5 N NaOH for 30 min16.
Structural changes on polyethylene surface
The Fourier Transform Infrared Spectroscopy(FTIR) and Scanning Electron Microscope(SEM) analysis of untreated polyethylene,UV irradiated polyethylene and samples of polyethylene films after biotic degradation for 30 days were studiedfor understanding structural changes.The change in the polyethylene surface and generation of new peaks wereobtained by FTIR spectroscopy (FTIR Bruker, Model: Tensor 27) from 400 to 4000 cm−1. Using the FTIR spectrum,carbonyl residue as carbonyl index and double bond index were measured on the basis of ratio of relative intensities of the carbonyl band (1715 cm−1) and double bond band (1650 cm−1) to that of methylene scissoring band at 1460 cm−1. The bacterial growth and structural changes on polyethylene was checked by SEM analysis as per the method given byTribedi and Dey17.
Results and discussion
The hydrocarbon contaminated soil from the coal fired Thermal Power Plant, Bathindaused for screening polyethylene degrading bacterial communitywas slightly alkaline with pH 8.10±0.08and electric conductivity of 556± 1.8 µS/cm.The total petroleum hydrocarbon content present in contaminated soil affected the physicochemical properties18. The soil sample had bacterial population of 3.11× 109 CFU/g (Figure 1).
 |
Figure 1: (a) Hydrocarbon contaminated soil from Thermal power plant for polyethylene degrading bacteria (b) Viable bacteria from hydrocarbon contaminated soil on nutrient agar plates. |
The 16s rDNA sequencing of native bacterial community from hydrocarbon contaminated soil near coal fired thermal power plant identified predominant bacterialgenera as Steroidobacter, Flavisolibacter, Planctomyces, Balneimonas, Gemmata, Alicyclobacillus, Lactobacillus, Mycobacterium, Geodermatophilus, Prevotella, VirgisporangiumandAdhaeribacter.The bacterial populationsin the hydrocarbon contaminated soil were able to grow using polyethylene as sole carbon and energy source. It was clear from the protein concentration at 5 days with an increase of 3.69 times than 0 h. The continuous increase in protein concentration over time from the colonization of microbial populationson the polyethylene surface. The increase of protein concentration was 12.93 times and 25.98 times at 15 days and 30 days respectively than the inoculation. But the increase in biomass growth was declined to 44 percent in 10th day than 5th day (Graph 1). The increase was 2.44 times in the next 5 days which was again reduced to 59 percent in 20th day of biodegradation study. Further thepercent increase was reduced to 9.69 percent in 30th day of incubation.
The biomass growth using polyethylene as carbon source and biodegradation was confirmed from the 12.85± 0.16 percent weight loss of waste polyethylene bags in 30 days. The range of weight loss in polyethylene after biodegradation of 30 days was from 12.67 to 13.05 percent. This weight loss of polyethylene by bacterial community from hydrocarbon contaminated soil from coal fired thermal power plant was higher than LDPE biodegradation in 60 daysby Pseudomonas aeruginosa (ISJ14)from waste dumping site19. The polyethylene degradation was increased by different microbes than pure culture20.The microbial populations present in the hydrocarbon contaminated sites hadhigh biosurfactant production capabilityandpresence of alkane hydroxylase gene for competent biodegradation21.The degradation of polyethylene was due to interaction between differentpolyethylene degrading microorganisms22.
The UV irradiation increased the bacterial biomass (protein concentration) to 61.89 percent than untreated polyethylene films in 5 days and 33.82 percent in 30 days of degradation. The protein concentration of bacterial community from utilizingUV treated polyethylene as carbon source was 24.30 and 38.82 times higher in 15th day and 30th day of degradation respectively than the day of inoculation. The initial growth was also higher in UV treated polyethylene with 6.67 times in 5 days of inoculation. The rate of growth in UV treated polyethylene was doubled in 10 days which was reduced by 65.56, 24.45, 15.41 and 11.2 percent respectively for 15, 20, 25 and 30 -days incubation period.The UV exposure of waste polyethylene bags improved initial growth and more bacterial colonization on polymer surface.
The increase in biomass growth of bacterial community from hydrocarbon contaminated soil utilizing UV irradiated polyethylene as carbon and energy source was confirmed with biodegradation of 15.81± 0.32 percent weight loss of waste polyethylene bags in 30 days. The polyethylene after biodegradation had a weight loss ranged from 15.43 to 16.22 percent in 30 days.The UV irradiation on bacterial biodegradation of polyethylene bags was statistically found highly significant at P value <0.01 by one way ANOVA with Post- hoc Tukey test.The biomass growth was also found significant (P value <0.01) with the UV treatment of polyethylene bags.
The UV irradiation increased the carbonyl index and caused significant alteration on the polymer structure that favored the enhanced microbial attachment on polymer surface. The increased degradation of polyethylene was also reported by Jeon and Kim23 and Montazer and coworkers 24
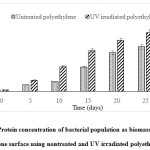 |
Graph 1: Protein concentration of bacterial population as biomass indicator on olyethylene surface using nontreated and UV irradiated polyethylene films. |
The structural modifications of UV treated polyethylene was clear from FTIR spectra. FTIR spectra of nontreated polyethylene films and UV irradiated films is shown in Graph 2. The infrared spectrum of the waste polyethylene bags after photooxidation (UV irradiation for 144h) showed a peak at 1716 cm−1 and 1055 cm−1 representing carbonyl groups (-C=O) and ether groups (-C-O-C-) respectively. The generation of additional peaks at 3807 cm-1, 3420 cm-1, 2362 cm-1, 2121 cm-1 and 1917 cm-1 after 144 h of UV irradiation also confirmed the structural changes in the polymer. The functional groups identified in the FTIR spectra were indicator precursors in the photochemical reactions of the polyethylene25. The prior UV pretreatments of polyethylene increased surface hydrophilicity by formation of additional carbonyl groups.Theradical was formed initially from absorption of UV-radiation on polyethylene and furtherto hydro-peroxide formation and then terminal carbonyl groups26.
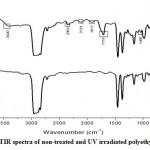 |
Graph 2: FTIR spectra of non-treated and UV irradiated polyethylene films. |
The carbonyl index showed the oxidation of polyethylene by UV radiation from the carbonyl species in the FTIR spectra.The UV pretreatment of the polyethylene for 144 h enhanced the ester carbonyl index and keto carbonyl index by 7.97 and 9.49 percent respectively.The increase in carbonyl index from UV treatment was due to photo-oxidative reaction of plasticizer and stabilizers present in polyethylene that later leads to chain scission27.
The UV irradiation of waste polyethylene bags also increased the brittleness of the polyethylene, surface roughness, cracks and depressions on the polymer surface as clear from scanning electron microscopy (SEM) images (figure 2).The structural changes on the UV irradiated polyethylene enhanced bacterial consortium from hydrocarbon contaminated soil to improve degradation by 22.80 percent higher than nontreated waste polyethylene films in vitro.
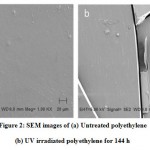 |
Figure 2: SEM images of (a) Untreated polyethylene (b) UV irradiated polyethylene for 144 h |
The SEM images after 30days biodegradation study was also confirmed the bacterial biodegradation of polyethylene (figure 3).The adherence of microorganisms on the surface of plastics followed by the colonization of the exposed surface are the major mechanisms involved in the microbial degradation of plastics 28. The microbes adhered to the surface increased biodegradation and cause cracks and cavities 29.
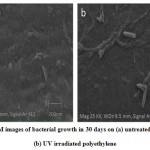 |
Figure 3: SEM images of bacterial growth in 30 days on (a) untreated polyethylene (b) UV irradiated polyethylene. |
The decrease in pH in the growth media with polyethylene evidenced the metabolism and the biodegradation process in four weeks period by bacterial populations from hydrocarbon contaminated soil. This was also in accordance with reports of Das and Kumar 30.
The weight loss of untreated polyethylene without pro-oxidant additives and oxidative pretreatmentby UV irradiation of polyethylene confirmed the biodegradation potential of bacterial community from hydrocarbon contaminated soil.The bacterial populations in the community from hydrocarbon contaminated soil had the polyethylene degrading ability from surface adhesion, bacterial proliferation and exopolysaccharide production 31. The interactions among bacterial populations in the communitywith different oxidative enzymes resulted in higher polyethylene biodegradation32,33.
The improvement in weight loss of the waste polyethylene byUV treatment and biotic degradation confirmedthe synergistic effect from photooxidation by UV radiationand biodegradation by bacterial communityfrom hydrocarbon contaminated soil24. The similar results were also reported by Albertsson et al. 26 and Esmaeili et al.34.
The UV light exposureinduced the photooxidation of the polymer and generated the free radicals that facilitate the microbial attack26, 35,36.The photooxidation by UV irradiation increased carbonyl bond and terminal double-bond index which in turn increased microbial utilization of carbonyl residues17,37,38.The biodegradation was initiated with adherence of microbes on surface of polyethylene39. The biodegradation of these recalcitrant pollutants was also enhanced by the surfactants produced by the microbes which increase bioavailability40.The enzymes present in the polyethylene degrading microorganisms from hydrocarbon contaminated soil cleave the polymer chain into monomers and smaller fragments that can be easily used up by the microorganism31,41. This depolymerization process with intracellular and extracellular depolymerases helped microbes from hydrocarbon contaminated soil to utilize polyethylene as carbon and energy sources 23, 42,43.The products wereeasily metabolized by microbes via the β-oxidation pathway and citric acid cycle44. .The biodegradation efficiency was improved in UV irradiated polyethylene and with microbial communities resulted in more microbial colonization and synergistic effect of photooxidation and biodegradation 26,45,46. Microbial biodiversity inhabiting contaminated sites have already proved high efficiency to degrade polyethylene. The nature and type of polyethylene, types of additive present in the polymer and extent of prior photooxidation pretreatment play a crucial role in biodegradation47.
Conclusion
The hydrocarbon contaminated soil from coal fired thermal power plant wasa good habitat for different efficient bacterial populations capable of degrading polyethylene wastes. The prior UV irradiation resulted in increased bacterial adherence, colonization andsynergism in polyethylene biodegradation. The combination of photo-oxidation and biodegradation helps in easy metabolism of polyethylene and in plastic waste management without any ecological impacts.
Acknowledgments
The authors are grateful to Central University of Punjab, Bathinda (India) for providingfacilities to undertakethe present work.The authors specially thank Central Instrumentation Facility, Central University of Punjab for FTIR and SEM analysis. The first author thanks University Grants Commission for the Junior Research Fellowship (UGC-JRF).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Uddin M.A., Koizumi K., Murata K., Sakata Y. Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Degrad. Stab. 1997; 56(1): 37-44.
CrossRef - Raaman N., Rajitha N., Jayshree A., Jegadeesh, R. Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around Chennai. Acad. Indus. Res. 2012; 1(6): 313-16.
- Central Pollution Control Board (2018). Life cycle assessment (LCA) study of Plastics packaging products.<https://cpcb.nic.in/uploads/plasticwaste/LCA_Report_15.05.2018.pdf >. Accessed 2020 December 25.
- Azubuike C. C., Chikere C. B., Okpokwasili G. C. Bioremediation techniques–classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016; 32(11): 1-18.
CrossRef - Albertsson A. C., Karlsson S. Aspects of biodeterioration of inert and degradable polymers. Biodeterior. Biodegrad. 1993;31(3):161–70.
CrossRef - Yoon M G., Jeon H. J., Kim M. N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. Bioremed. Biodegrad. 2012; 3(4): 1-8.
- Deepika S., Jaya M. R. Biodegradation of low density polyethylene by microorganisms from garbage soil. Exp. Biol. Agric. Sci. 2015; 3(1): 1-5.
- Gu J.D. Microbial colonization of polymeric materials for space applications and mechanisms of biodeterioration: A review. Biodeter. Biodegr. 2007; 59(3): 170–79.
CrossRef - Esmaeili A., Pourbabaee A. A., Alikhani H. A., Shabani , Esmaeili, E. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillusxylanilyticus and Aspergillus niger in soil. Plos one. 2013; 8(9): e71720.
CrossRef - Kyrikou I., Briassoulis D., Hiskakis M., Babou E. Analysis of photo-chemical degradation behavior of polyethylene mulching film with pro- oxidants. Degrad. Stab. 2011; 96(12): 2237-52.
CrossRef - Kolvenbach B. A., Helbling D. E., Kohler H. P. E., Corvini P. F. Emerging chemicals and the evolution of biodegradation capacities and pathways in bacteria. Opin. Biotechnol. 2014; 27: 8-14.
CrossRef - Singh J., Gupta K. C., Shrivastava A. Isolation and identification of low density polyethylene (LDPE) degrading bacterial strains from polythene polluted sites around Gwaliorcity (MP). Global. Biosci.2015; 4(8): 3220-28.
- Hadad D., Geresh S., Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillusborstelensis. Appl. Microbiol. 2005; 98(5): 1093-100.
CrossRef - Konduri M. K. R., Anupam K. S., Vivek J. S., Rohini Kumar D. B., Lakshmi Narasu M. Synergistic effect of chemical and photo treatment on the rate of biodegradation of high density polyethylene by indigenous fungal isolates. J. Biotech. Biochem. 2010; 6(1): 157–74.
- Gilan I., Hadar Y., Sivan A.,Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcusruber. Microbiol. Biotechnol. 2004; 65(4) 97–104.
CrossRef - Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J.Protein measurement with the Folin phenol reagent. Biol. Chem. 1951; 193 (1): 265-75.
CrossRef - Tribedi P., Dey S. Pre-oxidation of low-density polyethylene (LDPE) by ultraviolet light (UV) promotes enhanced degradation of LDPE in soil. Monit. Assess.2017; 189 (12): 1-8.
CrossRef - Masakorala K., Yao J., Chandankere R., Liu H., Liu W., Cai M., Choi M. M. A combined approach of physicochemical and biological methods for the characterization of petroleum hydrocarbon-contaminated soil. Sci. Pollut. Res.2014; 21(1): 454-63.
CrossRef - Gupta K.K., Devi D. Characteristics investigation on biofilm formation and biodegradation activities of Pseudomonas aeruginosa strain ISJ14 colonizing low density polyethylene (LDPE) surface, Heliyon 2020; 6(7):e04398.
CrossRef - Yin C. F., Xu Y., Zhou N. Y. Biodegradation of polyethylene mulching films by a co-culture of Acinetobacter sp. strain NyZ450 and Bacillus sp. strain NyZ451 isolated from Tenebrio molitor Int. Biodeterior. Biodegradation. 2020; 155(1): 105089
CrossRef - Lima S. D., Oliveira A. F., Golin R., Lopes V. C. P., Caixeta D. S., Lima Z. M., Morais, E. B. Isolation and characterization of hydrocarbon-degrading bacteria from gas station leaking-contaminated groundwater in the Southern Amazon, Brazil. J. Biol. Sci. 2020; 80(2): 354-61.
CrossRef - Tribedi P., Sarkar S., Mukherjee K., Sil A. K. Isolation of a novel Pseudomonas sp from soil that can efficiently degrade polyethylene succinate. Sci. Pollut. Res.2012; 19(6): 2115-124.
CrossRef - Jeon H. J., Kim M. N. Degradation of linear low density polyethylene (LLDPE) exposed to UV-irradiation. Polym. J. 2014; (52):146–53.
CrossRef - Montazer Z., Habibi-Najafi M. B., Mohebbi M., Oromiehei, A. Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. Polym. Environ. 2018; 26(9): 3613-25.
CrossRef - Ojeda T. F. M., Dalmolin E., Forte M. M. C., Jacques R. J. S., Bento F. M., Camargo F. A. O., Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Degrad. Stab. 2009; 94(6) 965–70.
CrossRef - Albertsson A. C., Andersson, S. O., Karlsson, S. The mechanism of biodegradation of polyethylene. Polym. Sci.1987; 18(1): 73-87.
CrossRef - Martínez-Romo A., González-Mota R., Soto-Bernal J.J., Rosales-Candelas I. Investigating the Degradability of HDPE, LDPE, PE-BIO, and PE-OXO Films under UV-B Radiation. J. Spectrosc. 2015 (2015). https://doi.org/10.1155/2015/586514.
CrossRef - Tokiwa Y., Calabia B. P., Ugwu C. U., Aiba S. Biodegradability of plastics. J. Mol. Sci. 2009; 10(9): 3722-42.
CrossRef - Volke-Sepulveda T., Saucedo-Castanede G., Gutierrez-Rojas M., Manzur A., Favela-Torres E. Thermally treated low density polyethylene biodegradation by Penicillium pinoohilum and Aspergillus niger. Appl. Polym. Sci. 2002; 83: 305–14.
CrossRef - Das M. P., Kumar S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech. 2015; 5(1): 81-86.
CrossRef - Sivan A., Szanto M., Pavlov V. Biofilm development of the polyethylene-degrading bacterium Rhodococcusruber. Microbiol. Biotechnol. 2006; 72(2): 346–352.
CrossRef - Peixoto J., Silva L. P., Krüger R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. Hazard. Mater. 2017; 324(1): 634–644.
CrossRef - Skariyachan S., Patil A. A., Shankar A., Manjunath M., Bachappanavar N., Kiran S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018; 149(1): 52–68.
CrossRef - Esmaeili A., Pourbabaee A. A., Alikhani H. A, Shabani F., Kumar L. Colonization and Biodegradation of Photo-Oxidized Low-Density Polyethylene (LDPE) by New Strains of Aspergillus sp. and Lysinibacillus sp.Bioremediat. J. 2014; 18(3): 213-26.
CrossRef - Ammala A., Bateman S., Dean K., Petinakis E., Sangwan P., Wong S., Leong K. H. An overview of degradable and biodegradable polyolefins. Polym. Sci. 2011; 36(8): 1015-49.
CrossRef - Ranjan V.P.; Goel S. Degradation of Low-density polyethylene film exposed to UV radiation in four environments. Hazard. Toxic Radioact. Waste. 2019; 23: 04019015
CrossRef - Gajendiran A., Krishnamoorthy S., Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016; 6(1): 52.
CrossRef - El-Sayed M. T., Rabie G. H.., Hamed E. A. Biodegradation of low-density polyethylene (LDPE) using the mixed culture of Aspergillus carbonarius and A. fumigates. Dev. Sustain. 2021; 23(1): 1-29
CrossRef - Yabannavar A., Bartha R. Biodegradability of some food packaging materials in soil. Soil Biol Biochem.1993; 25:1469–1475.
CrossRef - Viramontes-Ramos S., Portillo-Ruiz M. C., Ballinas-Casarrubias M. D. L., Torres-Muñoz J. V., Rivera-Chavira B. E., Nevárez-Moorillón G. V. Selection of biosurfactan/bioemulsifier-producing bacteria from hydrocarbon-contaminated soil. J. Microbiol.2010; 41(3): 668-75.
CrossRef - Ghatge S., Yang Y., Ahn J. H., Hur H. G. Biodegradation of polyethylene: a brief review. Appl. Biol. Chem. 2020; 63: 1-14.
CrossRef - Lau A. K., Cheuk W. W., Lo K. V. Degradation of greenhouse twines derived from natural fibers and biodegradable polymer during composting. Environ. Manage.2009; 90(2): 668–71.
CrossRef - Dey U., Mondal N.K., Das K., Dutta S. An approach to polymer degradation through microbes. IOSR Journal of PharmacyIOSRPHR 2012; 2:385–388.
CrossRef - Restrepo‐Flórez J.M., Bassi A., Thompson M. R. Microbial degradation and deterioration of polyethylene – a review. Biodeterior. Biodegrad. 2014; 88(1): 83–90.
CrossRef - Orr I. G., Hadar Y.,Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcusruber. Appl. Microbiol.Biotechnol. 2004; 65(1): 97–104.
CrossRef - Abrusci C.,Pablos J.L., Marín I.,Espí E., Corrales T., Catalina F. Comparative effect of metal stearates as pro-oxidant additives on bacterial biodegradation of thermal- and photo-degraded low density polyethylene mulching films. Biodeterior. Biodegrad. 2013; 83: 25–32.
CrossRef - Montazer Z., Habibi Najafi M. B., Levin D. B. Challenges with verifying microbial degradation of polyethylene. Polymers.2020; 12(1): 123
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






