How to Cite | Publication History | PlumX Article Matrix
Adsorption of Azo Dyes Using Biochar Prepared from Regional Crop Waste Material
Shridhar K. Jadhav* and Sanjaykumar R. Thorat
and Sanjaykumar R. Thorat
School of Environmental and Earth Sciences, Kavayitri Bahinabai Chaudhari North Maharashtra University, Jalgaon 425001, Maharashtra, India
Corresponding Author E-mail: profskjadhav@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2973
ABSTRACT:
Adsorption of dyes and a colorant using biochar is an emerging technology with environment-friendly and cost-effective applications. In this study, we investigated the adsorption isotherms of Brilliant Blue (BB) and Congo Red (RR) using Biochars prepared from regional plant waste of the Musa acuminata stem at 4500C (BSB 450). A batch experiment study for BB and CR with its initial concentration (1-240 mg/l) contact time (30—240 min) pH (3-10) and dose range (25-450 mg/l) at temperature 303K was carried out. The experimental data follows the Freundlich isotherm for BB and Langmuir isotherm for CR. As per our results adsorption isotherm equilibrium data shows the highest adsorption of BB onto BSB 450 is 175.57 mg/g and CR is 135.15 mg/g in Dubinin–Radushkevich model. The study also shows that BSB 450 is a cost-effective and environment-friendly adsorbent that was used for the treatment of dyes containing effluents like a tannery, textile, and dye waste producing industries.
KEYWORDS: Adsorption Isotherm; Brilliant blue; Biochar: Azo dyes; Musa acuminata; Congo red
Download this article as:| Copy the following to cite this article: Jadhav S. K, Thorat S. R. Adsorption of Azo Dyes Using Biochar Prepared from Regional Crop Waste Material. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Jadhav S. K, Thorat S. R. Adsorption of Azo Dyes Using Biochar Prepared from Regional Crop Waste Material. Biosci Biotech Res Asia 2022;19(1). Available from: https://bit.ly/3uukk0A |
Introduction
Effluents containing Dyes from textile, tannery, printing, and paper industry it is environmentally toxic, and considering major water body pollution worldwide; they exhibit adverse effects on humans, organisms, and nature. For colorant contained wastewater before its discharge on water bodies, it requires to give proper treatment. For wastewater treatment now various physical, biological, chemical treatments are available but adsorption is most preferable and usable1 . The key pollutants in effluent from industry can significantly fluctuate and are usually categorized by excessive amounts of constant substances, for example, organics, dyes, toxicants, surfactants, inhibitory compounds, chlorinated compounds, salts, and heavy metals, in addition to coloured dyes are the extremely challenging components of the wastewater5,8,20. In the traditional industrial treatment of effluent, common ways applied are physical, coagulation, chemical precipitation, biological treatment, electrochemical destruction, flotation, etc5,20. In the leather tanning process, a huge amount of highly toxic azo dyes, chemicals such as chrome salts, vegetable, and synthetic tannins, phenolic compounds, surface-active compounds, pesticides, and sulfonated oils, are being used to convert the rawhide/skins into commercial leather or leather products7,15. Effluents from the printing, textile, cosmetics, dyeing, food colouring, and papermaking industries are containing huge amounts of dyes. This coloured wastewater when mixed with groundwater and surface water system, will be creating serious effects on human health and the environment12,13,19. Dyes containing wastewater mainly resulting allergic diseases, irritation, cancer, and mutation13. Last few years, several tools have been developed and used for the elimination of the dye pollutants from industrial wastewater: including adsorption, coagulation/flocculation, enhanced oxidation practices, ozonation, membrane purification, and biological treatment5,18,18.
Wastewater treatment in a developing country is an economic and sustainable challenge6 because of the feasibility and cost of treatment, due to these, it requires low-cost and less complicated techniques. In the last decade, millions of researchers have been working on an environmentally friendly and cost-effective technology suitable for the industry. In this study, we are concerned with the adsorption technique with biochar produced from the agricultural wastes of Musa acuminata stem. Musa acuminata is native to the hot, tropical regions of Southeast Asia and is also common in Brazil, China, India, Ecuador, Colombia, and Venezuela10,16,17. Adsorption of pollutants from the effluent is one of the best-fitted and cost-effective as well as eco-friendly techniques for wastewater and dyes removal. In tannery effluent mainly the concentration of dyes is much high, so we are applying the adsorption technique for dyes adsorption by using natural adsorbent Biochar prepared from regional plant waste material.
Material and Methods
The material used in the present study is azo dyes Congo red (CR) (molecular weight is 696.665g/mol and formula-C32H22N6Na2O6 S2) and Brilliant Blue (BB) (molecular weight 792.85g/mol and formula- C37H34N2Na2O9S3). The used BB and CR solutions for this study were prepared from the stock solutions of 1000 mg/L. The additional chemicals were used in the research were also analytical grade and purchased from Merck. The Musa acuminata stem was collected from the farm near the Kavayitri Bahinabai Chaudhari North Maharashtra university campus Jalgaon, Maharashtra.
Preparation of Biochar
The air-dried fresh Musa acuminata stems are collected from the farm near the Kavayitri Bahinabai Chaudhari North Maharashtra University campus in the Jalgaon district Maharashtra. Collected Musca acuminata plant stem biomass was washed with distilled water for the elimination of dirt and unwanted particles; then air-dried for 24 hours after that it dried in a vacuum oven for 1 hour at 4500C temperature. oven-dried biomass cut into small pieces, then wrapped with aluminium foil perfectly. In wrapped foil a fine small pin-sized hole was drilled for oxygen presence. Finally, this biomass was placed in a muffle furnace (Lab Star make in India CAT No-LSI 031) Ramp set at 50 C for 4500 C temperature for 6 hrs. at atmospheric pressure. The biomass was grind in the mixer and sieved to its powdered material at <250µ particle size. The prepared biochar of Musa acuminata stem was named BSB-450 and used for the batch adsorption study.
Batch experiment for adsorption of azo dyes
The adsorption trials of the azo dyes namely Congo red (CR) and brilliant blue (BB) have been assessed in the batch method in the 100 mL flask. The control studies were conducted with 20 mg/L of 25 mL volume of both BB and CR dyes with the addition of 25 to 450mg/L adsorbent at pH 3.0. Moreover, these BB+BSB-450 and CR+BSB-450 mixture was shacked thoroughly by using an orbital shaker (Steelmet Novatech Model Table-Top) at 150 rpm for 1hr. The mixture of both BB+BSB-450 and CR+BSB-450 was then filtered through a Whatman’s filter paper which size is 42µ and the filtered solutions were used to evaluate the removal capacity of BSB-450. Analyzing the concentration of BB and CR was done by taking an absorption using UV-spectroscopy (SL-159, Elico, India). Batch study we used the concentration range of BB and CR is (1-260 mg/L), pH of BB and CR (3-10), and Dose of BSB-450 (25-450 mg/L). to estimate the highest removal efficiency of BSB 450 laboratory conditions.
Characterisation of BSB 450
Physical and chemical characteristic of Musa acuminata stem biochar (BSB 450) analysed in the labortory showing the following results presented in table no-1. Yield of the Biochar calculated by:
The pH is measured using a pH meter (elico model LI 120). The pH meter is calibrated with buffer solutions pH 4, pH 7 and pH 9. The prepared BSD 450 is dissolved in double distilled water (distilled water of MILLI-Q model Direct 8 from Millipore) and shaken with a shaker at 150 rpm for 1 hour. The pH of BSB450 major by the standard procedure it Shows pH 8.61.
Electrical conductivity is measured using a digital conductivity meter (Elico model no- CM 180). The conductometer is calibrated with a 0.1NKCL solution. Prepared BSB450 is added to double distilled water and shaken in shaker at 150 rpm for 1 hour. Then the electrical conductivity is measured according to the standard procedure, which is 42.26 mS/cm. Table No. 1. In the adsorption study, the surface area of the adsorbent plays an important role in the analysis of BSB 450 by field emission scanning electron microscopy (FESEM, S-4800, Hitachi, USA). The surface of BSB 450 is soft and has a porous structure, which is helpful for the adsorption of pollutants or dyes. The after image of BSB 450 shows a coarse surface with adsorbed dye folds and some pores. Consequently, the FESEM image shows the above-mentioned changes, resulting in a strong affinity of biochar for the adsorption of azo dyes.
 |
Figure 1: FESEM images for BSB-450 adsorbent (a) before and (b) after adsorption. |
Table 1: Physical and chemical characteristics of the Banana stem biochar (BSB)
| Samples | BSB-450 |
| Yield (%) | 39.49% |
| Ash (%) | 47.17% |
| Volatile Matter (%) | 4.64% |
| Fixed Carbon (%) | 48.19% |
| pH in ultrapure water (1:5 w/w) | 8.61 |
| Electrical Conductivity (EC) | 48.26mS/cm |
| Cation Exchange Capacity (CEC) | 132.7cmol-kg-1 |
| Organic carbon (%) | 19.98% |
| Organic Matter (%) | 34.44% |
| Metals in extract | |
| Sodium (Na) | 205ppm |
| Potassium (K) | 2551ppm |
| Calcium (Ca) | 160.32ppm |
| Magnesium (Mg) | 729.6ppm |
Result and Discussion
pH study
Brilliant blue dyes and Congo red adsorption study carried at the range of pH 3-10 in these mainly the adsorption pattern shows the higher adsorption at pH 3 means at the high acidic condition the prepared biochar work much more effectively. BB adsorption at pH 3 is 98.52 percent after pH 3 it is continuously decreasing from pH 4 to 10 it shown in figure 2.
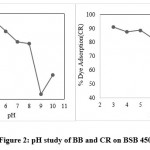 |
Figure 2: pH study of BB and CR on BSB 450. |
Adsorption of Congo red dyes at the pH range 3 to 10 shows the continuous decreasing order from pH 3 to 10. Maximum dyes removal on BSB 450 is at pH 3 it is 91.16 percent after pH 3 up to pH 10 it continuously decreases at pH 10 the removal percent is only 61.48. the maximum adsorption capacity of BSB 450 is at pH 3 so in all further studies, we use a pH range is 3.
Dose Study
In this batch study the adsorption of BB dyes on BSB 450 in the range of 25 mg to 225 mg at an interval of 25 mg dose. The batch experiment shows the highest result of adsorption or a dyes removal at 25mg after that the increases dose level from 25 mg to 225 mg its removal capacity continuously decreases the highest removal percent is 89.20 percent at 25 mg it shown in figure no 3.
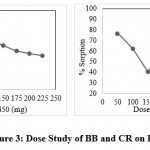 |
Figure 3: Dose Study of BB and CR on BSB450. |
Adsorption of CR dyes onto the BSB 450 batch experiment we used the dose in the range of 50mg to 450mg interval up to the 50mg to obtain data from the experiment showing the higher adsorption at 50mg is 76.67 percent dose after the increasing dose from 50mg to 450 its decreases so further study we use 50mg dose of BSB 450.
Time Study
In the Adsorption study contact time required is most important for the removal or adsorption. In these batch experiments, we use the time interval of 30 minutes for each in the set-up time from 30 minutes to 240 minutes time interval study the results obtained is the maximum adsorption at a time 60 minutes means a one hour. The obtained data in figure no 4 shows at 1 hour 98.95 percent adsorption of BB on BSB450.
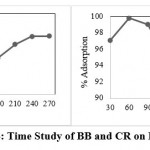 |
Figure 4: Time Study of BB and CR on BSB 450. |
Time study of CR on BSB 450 is showing the removal of the maximum dye at the range of 60 minutes. We study the time interval of 30 minutes from 30 minutes to 270 minutes variation to obtain maximum adsorption efficiency with time relation. In these batch experiments, the higher adsorption at a time interval of 60 minutes is 99.79 percent after the increasing time the efficiency of the adsorbent slightly decreases from 90 minutes to 150 minutes after that remain steady adsorption pattern shows BSB 450 in figure No 4.
Concentration study
Concentration study and adsorption capacity of BSB 450 observe for Brilliant blue dyes using the range from 1mg/L to 240mg/L. As per dose adsorption observation, we use the dose of 1g/L, BB dye adsorption onto the BSB-450 is shown in Figure 5. In this research study, it is observed that the sorption capacity of BSB 450 continuously increases from 1mg/g to 240mg/g obtained data shows it is 110.14mg/g to 217.26mg/g respectively. The adsorption capability of BSB 450 continuously rises from 1 to 240 mg/L for the synthetic solution, In the batch experiment observed the maximum adsorption capacity of BSB-450 was 217.27 mg/g during the removal of BB dye.
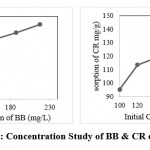 |
Figure 5: Concentration Study of BB & CR on BSB 450. |
CR dyes concentration study on BSB-450 investigated in the range from 1 mg/L to 240 mg/L laboratories prepared synthetic solution. In batch experiments, a dose of BSB-450 was used is 1 g/L in which is the maximum adsorption of CR on BSB-450 was observed. In the following Figure 5, the obtained data shows concentration versus adsorption of CR, in which the sorption at 1mg/L was 95.33 mg/g, and 120 mg/L to 200 mg/L was found to be 113.75, 118.56, 129.88, 135.29, 138.99, 135.69, and 124.33 mg/g. In these, the maximum sorption at 200 mg/L was found to be 138.99 mg/g.
Adsorption Isotherm
The theoretical models of adsorption isotherms of Freundlich, Langmuir, Temkin, and Dubinin-Radushevich were used and applied for the present adsorption study. These linear and non-linear equation models of adsorption isotherms are listed in Table5 No. 2. The estimation of the isotherm constant models plotted using the linear regression equation and the values used to determine the coefficient (R2) for BB and CR are presented in Table No. 3. For the study of BB and CR adsorption onto the BSB 450, the pH is 3, the dose of adsorbent BSB 450 is 25 mg, the contact time is 1 hour, and 303 K normal room temperature.
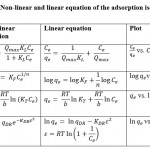 |
Table 2: Non-linear and linear equation of the adsorption isotherms5. |
Table 3: Adsorption isotherm constant Non-linear and linear equationparameter with coefficient regression adsorption of BB and CR onto BSB 450.
| Isotherm model | Parameters | Error Function | |||||
| Langmuir | qm (mg/g) | KL (L/mg) | R2 | χ2 | Δq | ||
| BB | 172.41 | 0.35 | 0.9185 | 41.11 | 16.8 | ||
| CR | 129.87 | 77 | 0.9938 | 4.28 | 15.43 | ||
| Freundlich | KF (mg/g)/(mg/L)n | 1/n | |||||
| BB | 6.57 | 5.45 | 0.9677 | 13549.73 | 98.41 | ||
| CR | 1.09 | 5.24 | 0.6906 | 56946.18 | 105.09 | ||
| Temkin | KT (L/g) | b (kJ/mol) | |||||
| BB | 18.78 | 8.92 | 0.9633 | 61.82 | 16.82 | ||
| CR | 4.1 | 2.42 | 0.9013 | 3.67 | 6.87 | ||
| Dubinin–Radushkevich | qDR (mg/g) | KDR (mol2/ KJ2) | E (KJ/mol) | ||||
| BB | 175.57 | 7.29 | 0.26 | 0.1735 | 32.61 | 14.70 | |
| CR | 135.18 | 8.5 | 0.24 | 0.4738 | 129.44 | 24.43 | |
Equilibrium factor (RL) its elaborate and show the characteristics of Langmuir isotherm model it is represents
The above (RL) values characterised the shape of isotherm its denote when 0<RL<1 is favourable=1 its Linier to corresponds>1 its unfavourable and whenever value of RL = 0 its irreversible. BSB 450 examination with BB and CR calculated RL values in between 0 and 1 that represent the favourable adsorption of dyes on to BSB450. the initial concentration of dyes used 1 to 240 mg/L. In the presented table no 3 of adsorption isotherm models values obtained from linear equation for BB shows in Langmuir graph value of KL (L/mg) is 0.35 and qm (mg/g) is 172.41 but the maximum adsorption shows in Dubinin-Radushkevich is175.57mg/g. in D-R model KL value estimated using intercept of linear plot is 7.29. as well the similarly the maximum Qmax adsorption of CR is found to in Dubinin- Radushkevich model it is 135.18mg/g and KL intercept calculated value is 8.5.
Dubinin-Radushkevich isotherm model helps to analyse physical and chemical nature of adsorption by estimating the (E) energy the equation is:
From the table no 2 constants KDR (mol2/kJ2) and qDR (mg/g) determined using the linear plot equation. E values shows the nature of adsorption when E value is below 8 kJ/mol physical adsorption process is overriding, E is in 8-16 kJ/mol then occurrence of ion exchange and the range 20-40kJ/mol, shows the chemical nature of adsorption (Gupta et al., 2013). In the present study the value of E(kJ/mol) for both CR and BB its less than 8 kJ/mol which is 0.24 and 0.26 kJ/mol Respectively so its conclude that the physical adsorption takes place in BB and CR onto the BSB 450.Present study used Freundlich, Langmuir, Temkin and Dubinin-Radushevich isotherm model from which is best applicable were assessed by using coefficient of determination (R2) data obtained of linear regression through isotherm equation. From these isotherm model (R2) value is much nearest to unity in Freundlich isotherm model for BB dye and in Langmuir isotherm model for CR dye onto the BSB450; the (R2 ) value are 0.9677 and 0.9938 respectively.
 |
Figure 6: Adsorption isotherm model graph of BB on BSB 450 a) Langmuir b) Freundlich c) Temkin d) Dubinin-Radushkevich |
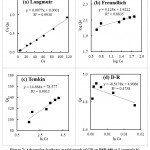 |
Figure 7: Adsorption isotherm model graph of CR on BSB 450 a) Langmuir b) Freundlich c) Temkin d) Dubinin-Radushkevich |
Present investigation Highest adsorption of BB and CR onto the BSB 450 is 175.57 mg/g and 135.18 mg/g respectively in the Dubinin-Radushkevich model. adsorption in comparison to the previous study is 50 to 100 times greater as demonstrated in table no 4 and 5.
Table 4: Adsorption of Congo red dyes on various adsorbent.
| Raw material For Biochar/(Adsorbent) | Adsorption mg/g | Reference |
| p-ECNFs | 100.5 mg/g | [19] |
| o-ECNFs | 74.2 mg/g | [19] |
| m-ECNFs | (47.3 mg/g | [19] |
| ECNFs-NH2 | 44.4 mg/g | [19] |
| ECNFs-COOH | 8.9 mg/g | [19] |
| ECNFs poly(phenylenediamine) grafted electrospun carbon nanofibers | 5.6 mg/g | [19] |
| Present Study | 175.57mg/g | Present Study |
Brilliant blue Remazol adsorption on to the PFPAC9 is 81.35% , Brilliant blue dyes adsorption on snail shell powder3 at a varying temperature form 298k to 338k its found to be highest 416.66 mg/g at 328k and low at 308k is 45.45 mg/g, in the present study it was highest (in a percent format) 96.5% .
Table 5 : Adsorption of Congo red dyes on various adsorbent
| Raw material For Biochar/(Adsorbent) | Adsorption mg/g | Reference |
| Coir pith | 6.7 | [4] |
| Bamboo dust | 101.9 | [09] |
| Coconut shell | 188.4 | [09] |
| Groundnut shell | 110.8 | [09] |
| Rice Husk | 237.8 | [09] |
| Straw carbon | 403.7 | [09] |
| Commercial activated carbon | 493.8 | [09] |
| Rice husk ash | 7.047 | [2] |
| Carbon slurry waste | 272.0 | [1] |
| Bael shell | 98.03 | [14] |
|
Musa Acuminata stem |
135.18 |
Present study |
BB and CR adsorption isotherm study obtained data which is model of isotherm is applicable estimated from the (R2) determination of coefficient obtained from regression isotherm equation. Studied isotherm model Langmuir, Freundlich, Temkin and Dubinin-Radushevich (R2) value is less than 1 for both the BB and CR azo dyes adsorption on to BSB 450 which unity closet to each other in the Freundlich model highest than all other model it is 0.9677 for BB and which is best fitted model of adsorption isotherm of brilliant blue for CR value of (R2) is the 0.9938 in Langmuir isotherm model respectively. As per data obtained the at a higher concentration range of 1mg/L to 240 mg/L BB and CR the isotherm model Dubinin-Radushkevich is low sorption.
Conclusion
Eco-friendly and cost-effective biochar derived from plant waste material of Musa Acuminate stem (BSB 450) was studied to investigate removal of azo dyes Brilliant Blue BB and Congo Red CR from synthetic aqueous medium by using adsorption isotherm models with batch experiment study. Experimental data suggest that maximum sorption capacity estimated for BB and CR in Dubinin-Radushkevich isotherm is 175.57mg/g and for CR is 135.18mg/g respectively at 303K temperature. Obtained experimental data showed the best fitted isotherm model from R2 value is for BB is Freundlich and for the CR is Langmuir. For sorption of BB and CR onto BSB 450 the involvements of external forces estimated from D-R isotherm model indicates the energy involved in batch sorption experiment is insignificant in sorption. Theses research work suggest the biochar prepared from regional plant waste material is dynamic potential to removal of BB and CR as well as its applicable environmental pollutant removal. BSB 450 is cost-effective material for remediation of wastewater containing dyes, as well adsorption by using biochar is sustainable and nothing harmful to environment techniques. For future study we suggested that Musa acuminata is available worldwide, but it has not been studied for adsorption and preparation of biochar, so it is need of todays to explore the study for removal of pollutant by biochar.
Acknowledgement
I Sincerely Gratitude towards Dr. Babasaheb Ambedkar Research and Training Institute for Providing a Dr. Babasaheb Ambedkar National Research Fellowship 2018 funded by Department of Social Justice and Special Assistance, Government of Maharashtra, India and also thankful to Research and Development Section and Director, School of Environmental and Earth Sciences, Kavayitri Bahinabai Chaudhari North Maharashtra University Jalgaon, Maharashtra for providing Teaching Associate Award (TAP) with all Research Facility.
Conflict of interest
There is no conflict of interest.
Funding source
We informed there is not available but we have Award letter No & Date: BARTI/Fellowship/BANRF-2018/19-20/3036. 30/06/2020
References
- Bhatnagar, A.K. Jain, M.K. Mukul, Removal of Congo red dye from water using carbon slurry waste, Environ. Chem. Lett. 2 (2005) 199–202. https://doi.org/10.1007/s10311-004-0097-0
CrossRef - K. Chowdhury, A.D. Sarkar, A. Bandyopadhyay, Rice husk ash as a low-cost adsorbent for the removal of methylene blue and congo red in aqueous phases, Clean 37 (2009) 581–59. https://doi.org/10.1002/clen.200900051 .
CrossRef - Ala‘a H. Al-ogaili and Muneer A. Al-Da’Amy Equilibrium and Thermodynamic studies of Adsorption of Remazol Brilliant Blue dye on snail shell powder 2020 IOP Conf. Ser.: Mater. Sci. Eng. 871 012036. https://doi.org/1088/1757-899X/871/1/012036 .
CrossRef - Namasivayam, D. Kavitha, Removal of Congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste, Dyes Pigments 54 (2002) 47–58. https://doi.org/10.1016/S0143-7208(02)00025-6 .
CrossRef - Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6:2335–2343. https://doi.org/1016/j.jece.2018.03.028
CrossRef - Dinesh Mohan, Ankur Sarswat, Yong Sik Ok, Charles U. Pittman, Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent A critical review, Bioresource Technology,Volume 160,2014,Pages191-202,ISSN0960-8524, https://doi.org/10.1016/j.biortech.2014.01.120 .
CrossRef - Dixit S, Yadav A, Dwivedi PD, Das M (2015) Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 87:39–49. http://dx.doi.org/10.1016/j.jclepro.2014.10.017
CrossRef - Ganpat B. More, Shridhar K. Jadhav and Sanjaykumar R. Thorat (2019): Development of a Novel Submerged Membrane Bioreactor (SMBR) for Treatment of Textile Wastewater. Ijrat,7,4,35-46 .
CrossRef - Kannan, N., Meenakshi Sundaram, M. Adsorption of Congo Red on Various Activated Carbons. A Comparative Study. Water, Air, & Soil Pollution 138, 289–305 (2002). https://doi.org/10.1023/A:1015551413378
CrossRef - Kumar, Nishant & Ved, Akash & Yadav, Ritu & Prakash, Om. (2021). A Comprehensive Review on Phytochemical, Nutritional, and Therapeutic Importance of Musa acuminate. International Journal of Current Research and Review. 13. 114-124. https://doi.org/10.31782/IJCRR.2021.13901
CrossRef - Mohd Azmier, Ahmad, Muhammad Aswar Eusoff, Peter Olusakin Oladoye, Kayode Adesina Adegoke, Olugbenga Solomon Bello, Statistical optimization of Remazol Brilliant Blue R dye adsorption onto activated carbon prepared from pomegranate fruit peel, Chemical Data Collections,28,2020, https://doi.org/10.1016/j.cdc.2020.100426
CrossRef - Namasivayam C, Muniasamy N, Gayatri K, et al (1996) Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour Technol 57:37–43. https://doi.org/10.1016/0960-8524(96)00044-2
CrossRef - Namasivayam C, Prabha D, Kumutha M (1998) Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour Technol 64:77–79. https://doi.org/10.1016/S0960-8524(97)86722-3
CrossRef - Rais Ahmad, Rajeev Kumar Adsorptive removal of congo red dye from aqueous solution using bael shell carbon Applied Surface Science 257 (2010) 1628–1633, https://doi.org/10.1016/j.apsusc.2010.08.111
CrossRef - Saxena, Gaurav & Chandra, Ram & Bharagava, Ram. (2016). Environmental Pollution, Toxicity Profile and Treatment Approaches for Tannery Wastewater and Its Chemical Pollutants. Reviews of environmental contamination and toxicology. 240. https://doi.org/10.1007/398_2015_5009 .
CrossRef - Simmonds NW, Shepherd K. The taxonomy and origins of culti-vated bananas. Bot. J. Linn. Soc. 1955; 55:302–12.https://doi.org/10.1111/j.1095-8339.1955.tb00015.x
CrossRef - Nayar, Nm. (2010). The Bananas: Botany, Origin, Dispersal. https://doi.org/10.1002/9780470527238.ch2 .
CrossRef - Srivatsav, Prithvi Bhargav, Bhaskar S. Shanmugasundaram, Vignesh Arun, Jayaseelan Gopinath, Kannappan P. Bhatnagar, Amit Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review Water 2020 12 12- 2073-4441 DO https://doi.org/10.3390/w12123561
CrossRef - Thamer BM, Aldalbahi A, Moydeen A M, et al (2019) Effective adsorption of Coomassie brilliant blue dye using poly (phenylene diamine) grafted electrospun carbon nanofibers as a novel adsorbent. Mater Chem Phys 234:133–145. https://doi.org/10.1016/j.matchemphys.2019.05.087
CrossRef - Walker GM, Hansen L, Hanna JA, Allen SJ (2003) Kinetics of a reactive dye adsorption onto dolomitic sorbents. Water Res 37:2081–2089. https://doi.org/1016/S0043-1354(02)00540-7
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.







