How to Cite | Publication History | PlumX Article Matrix
Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028)
Sridevi Veluru1*, Chandana Lakshmi V. V. Mahanti1, Narasimha Rao Medicherla2 and A. V. N. Swamy3
1*Department of Chemical Engineering (Biotechnology), Andhra University, Visakhapatnam - 17 India.
2Al-Ameer College of Engineering and Information Technology, Anandapuram, Gudilova, Visakhapatnam India.
3Department of Chemical Engineering, JNTU, Anantapur, India.
Corresponding Author E-mail: mahantilakshmi@yahoo.com
ABSTRACT: Phenolic compounds are hazardous pollutants that are toxic relatively at low concentrations. Accumulation of phenol creates toxicity both for flora and fauna. Because of its toxicity, there is a need to decontaminate the phenol-laden soils hence, bioremediation is a very useful alternative to conventional clean-up methods. The aim of this work was to study the effect of inoculum size and the influence of pH on phenol degradation by Pseudomonas desmolyticum. Phenol was degraded rapidly at pH values (6 to 9), but the maximum rate of phenol degradation by Pseudomonas desmolyticum was at pH 6. In contrast, the phenol degradation at pH (7, 8, and 9) were significantly lower, although phenol was totally depleted. Phenol was degraded at every inoculum size tested (1-10% v/v) but the maximum rate of phenol degradation was observed at 4% v/v in batch experimental system. These results are useful to understand the physiological and biochemical properties of Pseudomonas desmolyticum before its optimum use in environmental application and these data will assist in choosing the right phenol degrader for a changeable environment.
KEYWORDS: Biodegradation; Inoculum size; pH; Phenol; Pseudomonas desmolyticum.
Download this article as:| Copy the following to cite this article: Veluru S, Mahanti C. L. V. V, Medicherla N. R, Swamy A. V. N. Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotech Res Asia 2010;7(1) |
| Copy the following to cite this URL: Veluru S, Mahanti C. L. V. V, Medicherla N. R, Swamy A. V. N. Effect of Ph and Inoculum Size on Phenol Degradation by Pseudomonas Desmolyticum (NCIM 2028). Biosci Biotech Res Asia 2010;7(1). Available from: https://www.biotech-asia.org/?p=9685 |
Introduction
The massive increase in the synthesis of organic chemicals by man has led to the production of wide variety of compounds, some of which are xenobiotic. Their xenobiotic character means that their structures are not easily recognized by existing degradative enzymes and as a result they accumulate in the environment [1]. As they persist in the environment, they are capable of long-range transportation, bioaccumulation in human and animal tissue and biomagnifications in food chain. Phenol and its higher homology are aromatic molecules containing hydroxyl group attached to the benzene ring structure. The origin of phenol in the environment is both natural and industrial .Natural sources of phenol include forest fire, natural run off from urban area where asphalt is used as the binding material and natural decay of lignocellulosic material. Industrial sources such as oil refineries, chemical, petrochemical, pharmaceutical, metallurgical, pesticide products, paint and varnish industries, textile and also in the polymer industries like phenolic resins, bisphenol A, alkylphenols, caprolactums and adipic acid [2].The presence of phenol in water imparts carbolic odor to receiving water bodies and can cause toxic effects on aquatic flora and fauna [3]. It is lethal to fish even at relatively low concentrations of 5 – 25 mg L-1 [4]. Phenols are toxic to human beings and effects several biochemical functions [5].The concentration of phenols in waste waters varies from 10 to 300 mg L-1. Phenol is also a priority pollutant and is included in the list of EPA (1979) [6]. As a result, phenol – containing effluents have to be properly treated prior to discharge [7, 8, 9, 10, 11]. Efficient treatment methods are necessary to reduce phenol concentration in waste water to acceptable level, which is 5 ppm (USEPA).
Conventional methods of treatment for phenolic wastes have been largely chemical or physical methods like chlorination, advanced oxidation process [12], adsorption, solvent extraction, coagulation, flocculation, reverse osmosis, ozonation, photo catalysis, and electrolytic oxidation [13], but these processes have led to secondary effluent problems. Biological treatment for the bulk removal of these pollutants is therefore generally preferred. Biological degradation of phenol has been extensively studied using pure and mixed cultures [14, 15, 16, 17, 18] .Few studies have been carried out with the bacterium Pseudomonas desmolyticum in pure cultures [19] in which phenol is degraded via the meta-pathway [20].The success of bioremediation may depend on the availability of microbial strains that can mineralize high levels of phenol and withstand adverse conditions to compete under in situ conditions. An effective bacterial inoculum should be able to tolerate high levels of phenol while maintaining a high level of activity to provide efficient mineralization [21]. Understanding the physiological and biochemical properties of phenol degrading bacteria is required before optimum use of bacteria in environmental applications.
The biodegradation of phenol by Pseudomonas desmolyticum (NCIM 2028), a potential biodegradent of phenol has been investigated for its degrading potential under different operating conditions. Two variables of pH and inoculum size were used to identify the significant effects and interactions in the batch studied.
Methodology
Chemicals
Phenol (99% pure, chemical grade) 4-amino antipyrine and all other chemicals used were from Merck.
Source of organism
The microorganism Pseudomonas desmolyticum (NCIM 2028) was obtained from culture collection (NCL) Pune, India. The microorganism was maintained on a medium containing Beef extract: 1.0 g L-1, Yeast extract: 2.0 g L-1, Peptone: 5.0 g L-1, NaCl: 5.0 g L-1 and Agar: 20 g L-1 The pH of the medium was adjusted to 7.0 by adding 1N NaOH. It was stored at 320C for further use.
Growth determination
To study the extent of degradation, the cells were grown in a minimal salts medium with the following composition: Phenol 0.500 g L-1; K2HPO4, 1.5 g L-1; KH2PO4, 0.5 g L-1; (NH4)2SO4, 0.5 g L-1; NaCl, 0.5 g L-1; Na2SO4, 3.0 g L-1; yeast extract, 2.0 g L-1; Ferrous sulfate, 0.002 g L-1; CaCl2,0.002 g L-1 in conical flasks containing and inoculated with Pseudomonas desmolyticum (NCIM 2028) .The experimental studies were carried out in shake flasks with agitation at a rate of 120 rpm, temperature at 320C. Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Influence of pH of the medium on phenol degradation
Pseudomonas Cells were grown in MS medium with 500 mg L-1 of phenol at different pH values (6, 7, 8 and 9).This mixture was contained in 250 ml Erlenmeyer flasks. The cultures were placed on a shaker (120rpm) at 320C. At different times, growth and phenol degradation were measured.
Effect of inoculum size on phenol degradation
The effect of inoculum size (1 – 10% v/v) on phenol degradation was tested. Cells were grown as shake cultures at 320C in MS medium supplemented with 500mg L-1 phenol at pH 7 in 250 ml Erlenmeyer flask. At different times growth and phenol degradation were measured.
Estimation of phenol
Phenol was determined quantitatively by the Spectrophotometric method (DR/ 4000 V, Hach) using 4-amino antipyrine as the color reagent (λ max: 500nm) according to standard methods of analysis (22).
Growth determination
Bacterial growth was determined in terms of cell mass by measuring optical density at a wavelength of 500nm.
Results and Discussion
Biological treatment using Pseudomonas desmolyticum (NCIM 2028) was the most effective method for removal of phenol. It is also a time saving method compared to other conventional methods.
Influence of pH of the medium on phenol degradation
Four pH values from 6 to 9 were investigated in Figure 1. Phenol was degraded rapidly at pH 6. At this pH value, phenol degradation was high compared to other pH values. However, the phenol degradation at pH 7, 8, 9 was slower and phenol concentration decreased rapidly after 24 h inoculation. These results showed that Pseudomonas desmolyticum degraded more phenol per day at pH 6 than at any other pH value.
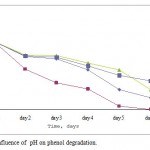 |
Figure 1: Influence of pH on phenol degradation.
|
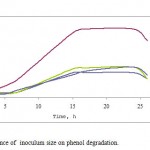 |
Figure 1: Influence of pH on phenol degradation.
|
Effect of inoculum size on phenol degradation
Phenol was degraded by Pseudomonas desmolyticum during all the inoculum sizes (1-10% v/v) tested in Figure 2. At 4% v/v the phenol concentration began to decrease rapidly after 5 h and reached 5mg L-1 after approximately 70 h. However, in cultures receiving lower inoculum densities, there was a progressive decrease of phenol concentration.
In addition, the rate of phenol degradation was tested. Cultures inoculated with 4%v/v inoculum size showed the highest rate of phenol degradation, while the cultures inoculated with a higher inoculum size showed a decrease in phenol consumption.
Phenols are commonly employed chemicals that are widely used in many industries, Because of their widespread use ,phenols are also major pollutants and are found in many industrial waste waters.Phenol can be toxic at low concentrations and cause taste and odour problems in drinking water at far low concentrations. It is lethal to fish even at relatively low concentrations of 5 to 25 mg/l Hence the removal of phenol from waste waters is of obvious interest.Biological degradation is generally preffered due to lower costs and the possibility of compleate mineralization. The biodegradation of phenol by pseudomonas desmolyticum(NCIM 2028),a potential biodegradent of phenol has been investigated for its degrading potential under different operating conditions.
References
- Singleton, I. Microbial metabolism of xenobiotics: Fundamental and applied research. Chem. Biotechnol. 1994, 59, 9-23.
- Paula, M.; Van Schei, Young, L.Y. Isolation and characterization of phenol degrading denitrifying bacteria. Environ. Microbiol. 1998, 64, 2432-2438.
- Ghadhi, S.C.; Sangodkar, U.M.X. Potentials of Pseudomonas cepacia PAA in bioremediation of aquatic wastes containing phenol. Proceedings of National Symposium Frontiers in applied and Environmental Microbiology. Cochin. 1995, 11-13.
- Nuhoglu, A.; Yakin, B. Modeling of phenol removal in a batch reactor. Proc. Biochem. 2005, 40, 233-239.
- Saha, N.C.; Bhunia, F.; Kaviraj, A. Toxicity of phenol to fish and aquatic ecosystem. Environ. Contam. Toxicol. 1999, 63, 195-202.
- Indu Nair, C.; Jayachandran, K.; Shankar Shashidhar. Biodegradation of phenol. African J. Biotechnol. 2008, 7, 4951-4958.
- Keith, L.H. Identification of organic compounds in unbleached treated kraft paper mill wastewaters. Env. Sci. Technol. 1976, 10, 555-564.
- Jungclaus, G.A.; Lopez-Avila, V.; Hites, R.A. Organic compounds in an industrial wastewater. A case study of their environment impact. Env. Sci. Technol. 1978, 12, 88-96.
- Parkhurst, B.R.; Bradshaw, A.S.; Forte, J.L. An evaluation of the acute toxicity to aquatic biota of a coal conversion effluent and its major components. Bull. Environ. Contam. Toxicol. 1979, 23, 349-356.
- Pfeffer, F.M. The 1977 screening survey for measurement of organic priority pollutants in petroleum refinery wastewaters. ASTM Spec. Tech. Publ. 1979, 181-190.
- Delfino J.J.; Dube, D.J. Persistent contamination of ground water by phenol. Environ. Sci. Health. 1976, A11, 345-355.
- Santiago Esplugas, Jaime Glimenez, Sandra Contreras, Esther Pascual, Miguel Rodriguez. Comparison of different advanced oxidation processes for phenol degradation. Water. Res. 2002, 36, 1034-1042.
- Arutchelvan, V.; Kanakasabai, V.; Elagovan, R.; Nagarajan, S.; Muralikrishna, V. Kinetics of high strength phenol degradation using Bacillus brevis. J. hazardous Mat. 2006, 129, 216-222.
- Kang, M.H.; Park, J.M. Sequential degradation of phenol and cyanide by a commensal interaction between two microorganisms. Chem. Technol. Biotechnol. 1997, 69, 226-230.
- Hugues, S.M.; Cooper, D.G. Biodegradation of phenol using the self-cycling fermentation (SCF) process. Biotechnol. Bio.Engg. 1996, 51, 112-119.
- Wang, K.W.; Baltzis, B.C.; Lewandowski. Kinetics of phenol biodegradation in the presence of glucose. Biotechnol. Bio. Engg. 1996, 51, 87-94.
- Ha, S.R.; Vinitnantharat, S.; Ozqki, H. Biodegradation by mixed microorganisms of granular activated carbon loaded with a mixture of phenols. Biotechnol. Lett. 2000, 22, 1093-1096.
- Chirwa, E.N.; Wang, Y.T. Simultaneous Chromium (VI) reduction and phenol degradation in an anaerobic consortium of bacteria. Wat. Res. 2002, 34, 2376-2384.
- Kalme,S.D.;Parshetti;Jadhar,S.U.;Govindwar,S.P.Biodegradation of benzidine based dye Direct Blue-6 by pseudomonas desmolyticum NCIM 2112.Bioresource Technology.2007, 98, 1405-1410.
- Sala-Trepat, J.M.; Murray, J.M.; William, P.A. The metabolic divergence in the meta- cleavage of catechols by pseudomonas putida NCIB 10015 Physiological significance and evolutionary implications. Env. Biochem. 1972, 28, 347-456.
- Shaw, K.W.; Lee, H.; Trevors, J. Effect of initial cell density, substrate concentration and temperature on pentachlorophenol degradation by Pseudomonas sp. UG-30. J. Chem. Technol. Biotechnol. 1997, 69, 107-113.
- American Public Health Association (APHA), American Water Works Association, Water Pollution Control Federation. Standard methods for the examination of water and wastewater. 17th Washington, D.C. American Public Health Association, 1989, 9-55 – 9-62.

This work is licensed under a Creative Commons Attribution 4.0 International License.





