How to Cite | Publication History | PlumX Article Matrix
Investigation on Identification, Density and Distribution of Zooplankton in Lar Reservoir in Iran
H. Abdollahpour Biria¹*, S. M. Salavatian² and A. Vahedi³
¹Department of Fisheries, Talesh Branch, Islamic Azad University, Talesh Iran.
²Talesh Branch, Islamic Azad University, Talesh Iran.
³Department of Agriculture, Astara Branch, Islamic Azad University, Astara Iran.
Corresponding Author E-mail: Abdollahpour51@yahoo.com
ABSTRACT: For optimum management, some limnological variables and zooplankton identification, density and distribution in Lar reservoir were studied over a two-year cycle( 2008 and 2009 ) during the ice-free seasons (May to October ).Samples were taken by simple plankton net (55ìm mesh size) from various depth layers (10-0 m, 20-10 m, 30-20 m) and were fixed with formalin %4. In the laboratory they were studied with inverted microscop. In this survey, we identified 21 genera in 3 zoopleanktonic orders. The order of Cladocera was predominant and comprised 55.81% of population annually. The predominate species and genera were Bosmina coregoni, Daphnia pulex, Daphnia longspina and embryonic Cladocerus. The order of Rotatoria comprised the second position with species of Asplanchna brightwelli , Polyarthera dolicopthera , Syncheata pectinata and Keratella cochlearis (percentage composition 37.71). Copepoda constitute 6.45 percentage compositions with five species and genera Cyclops vulgaris, C. smirnovi, Eucyclops sp., Arctodiaptomus acutilobatus and Copepoda Naupli.The mean density of for the sampling all monthly, mean of dominant Zooplanktonic density belonged to Cladocera order (32968.83±123.40 number/m3) while they were the least density in September by (6570 ±147.12 number/m3) Rotatoria were the second level with mean of 19074.66±115.39 number/m3 that the mean least of them with 9955.75±73.10 number/m3 were in September.Non-parametric statistical analysis based on Kruskal-Wallis and Mann-Whitney showed that there were significant differences between zooplankton abundance in different stations and months (P<0.05) but there was not any significant difference between different depths (P>0.05). Biological studies indicated that this reservoir has low potential to planktonic productivity and due to geographical location of this dam, remarkable temperature differences can be observed in different years especially in fall and winter, which is biologically important.
KEYWORDS: Zooplankton; Densityl; Distribution; Lar reservoir; Iran
Download this article as:| Copy the following to cite this article: Biria H. A, Salavatian S. M, Vahedi A. Investigation on Identification, Density and Distribution of Zooplankton in Lar Reservoir in Iran. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Biria H. A, Salavatian S. M, Vahedi A. Investigation on Identification, Density and Distribution of Zooplankton in Lar Reservoir in Iran. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9423/ |
Introduction
Investigation of food chains in the aquatic ecosystems is very important in point of view of the feeding habits of fishes. It is evident that live food aquaculture including production of fauna and flora is of particular significance in aquatic animal nutrition. In addition natural food are considered as rich sources of various chemical constituents such as protein, fat content, the necessary amino acids and enzymes which play a crucial role on fish growth 24. The importance of zooplankton in aquatic ecosystems is that they supply a nutritional source for fish larvae and a number of adult fish14,21,23.In addition to their socio-economic importance, reservoirs due to their high dissolved nutrient content and incoming organic load from the water shed areas, considered as productive systems that provide various aquatic animal populations with the nutritional substances 2.The main elements of these ecosystems include non-living factors (i.e. physico-chemical factors) and living factors (i.e. producers, consumers and decomposers) that are bound together through a highly complex biological structure. As secondary products, the zooplanktons constitute another ring of the food chine in aquatic ecosystems, which are constantly and actively present in different water resources. They are in turn, consumed by other members in the food chain, the nektons. Zooplanktons are major nutritional elements for a variety of aquatic animals ranging from larvae up to adult fish species. For certain reservoirs, such as the ones used for water supply, the understanding of zooplankton seasonality can be an important factor to be considered for quality management purposes 7.The Lar reservoir has an area of 1300 ha and is situated 55 km away in the north east of Tehran neighboring Polour, a village located 7 km away from the reservoir, which is itself fed by Polour River. The construction of the dam started in 1973 and ended in 1979. The dam is of earthen constructional material with clay core with length of 1170 meter. The height of the reservoir is 105 meter and the volume of the reservoir is 960 million m3. The Lar dam is located at 2569 m a.s.l. The Laar reservoir enjoys four distinct water shed areas and is fed by four rivers namely as “ Delichai” “Absefid” “ Kamardasht ” and “ Elarm” . There is a high seasonal fluctuation of water level. Based on recorded metrological data, the average daily air temperature is 6.5 0C with -30 0C and + 34.5 0C as the minimum and maximum temperature 5. Zooplankton identification and analysis play a significant role in their quality judgment, purification of sewage and industrial waste. It is also of importance in water management and control aimed at fish farming or swimming purposes. There has so far been no comprehensive study in “ Lar “ reservoir which made it necessary to launch an ongoing and comprehensive hydrological and hydrobiological investigation of “ the lake”. The present study focuses on zooplankton concentration and distribution as well as their role in the provision of food for fish. Meanwhile the study merely considers the fisheries capacity of the reservoir and its role as a recreational fishing site, with a view to assess its fisheries potential for brown trout through primary and secondary measurement of biomass.
Material and Method
Following the preliminary study, five stations were designated in the “Lar reservoir “(Base of the dam, the middle of the reservoir, Imam Pahnak, Absefid water inlet & Gezel Dareh) as shown in the map below (Fig.1 and Table 1).
 |
Figure 1. The location of the sampling station of zooplankton in the Lar reservoir.
|
Table 1: The location of the sampling stations and coordinates of Zooplankton in the Lar reservoir
| Coordinates | Station number | Station name |
| 35 55 06.7 N 54 50 30.3 E | 1 | Dam crest |
| 35 55 02.3 N 51 59 36.4 E | 2 | Middle of the lake |
| 35 55 37.6 N 51 54 10.5 E | 3 | Immam Pahnak |
| 35 55 56.2 N 51 56 41.6 E | 4 | Ab-sefid |
| 35 55 06.5 N 51 52 13.5 E | 5 | Gezel Dareh |
The monthly sampling of zooplanktons and limnological variable was carried over a two-year cycle (2008 and 2009) during the ice-free seasons (May to October). Since the Lar dam is located at 2569 m a.s.l., the surface of the reservoir completely ice-covered during early November to late April and sampling is difficult and sometimes virtually impossible. The zooplankton samples were collected using vertical zooplankton net (Judy Net, 30 cm diameter and 50 µm mesh size) hauls from various depths (10-0 m, 20-10 m, 30-20 m) in each station. The samples were preserved in 4% formalin soon after collection. For the quantitative analysis the zooplankton sub samples was enumerated in a counting chamber. Using upon homogenizing the samples by pipette in the laboratory, they were transferred to 5 ml containers and following sufficient sedimentation period were carefully examined and counted by invert microscope (Nikon).The samplings and examination of planktonic population were carried out using the fallowing references16,28 .The common taxonomic literature was used for identifying the Zooplankton 4,18,20 .The zooplankton density per cubic meter of water was determined in each of the stations and ultimately total density was specified. In order to analyze the data, the non-parametric Kurskal-Vallis and Man-Whitney tests was applied using SPSS software version 13.
Results
In all, 21 genera were found in the Laar reservoir, including 10 Rotifera, 6 Cladocera, 5 Copepoda. The highest abundance recorded occurred during May and in Cladocera amounting to 48050 ± 0.048 individual per cubic meter followed by Rotatoria with 24020±0.024 individual per m3. The Copepoda formed the lowest share of zooplankton diversity followed by Rotatoria which accounted for the bulk of zooplankton diversity with ten genera.The most prevalent zooplankton species noticed in this study were Cladocera (55.81%), Rotatoria (37.71%) and ultimately Copepoda (6.45%) (Fig. 2).The average zooplankton population at varying depths indicate a rising trend from spring and reaching its climax in May time, and then begin to decrease along with temperature decline and ultimately drops to its minimum level in winter time (Fig. 2).The highest abundance of zooplankton was observed in surface layer due to sunlight penetration particularly within 0-10 m depth range. The layer near to surface (> 0.5 m) revealed a less zooplankton population possibly because of adverse effect of suns rays. The deeper water layer (30 m) is a less populated area (Fig. 3).However Kurskal-vallis statistical test showed no significant difference in zooplankton abundance at different depth layers (sig =0.143,df=5,X2=8.251) (P>0.05).But Man-Whittny statistical test revealed significant difference in zooplankton abundance in different months of the year (sig=0.00,df=5, X2=58.64, P<0.05).There was also statistically significant difference in zooplankton abundance in various station within various monthly intervals (sig=0.00, DF=4, X2=21.49).
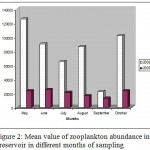 |
Figure 2: Mean value of zooplankton abundance in Lar reservoir in different months of sampling.
|
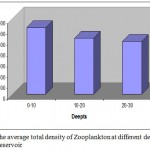 |
Figure 3: The average total density of Zooplankton at different depths in two years Lar reservoir.
|
Paired comparison of zooplankton population using non-parametric Man-Whittny test reveals that there was a statistically significant difference within different sites (Top section of dam-Absefid), (Top section of dam-Gezel Dareh), (middle of the lake-Absefid), (middle of the lake-Gezel Dareh), (Emampahnak-Gezel Dareh) and (Absefid-Gezel Dareh)( P<0.05).The mean zooplankton abundance in spring was 74730±0.023 individual per m3 which was the highest among the seasons.The genera Daphnia longspina, D.pulex, Bosmina coregoni B. longirostris, Macrothrix laticornis and Cladocera emberyoni belonging to Cladocera were dominant in spring whit mean abundance of 48050±0.048 individual per cubic meter. Nevertheless, during autumn the population of zooplankton was less than that of spring and the abundance of Cladocera and Rotatoria within the overall population was higher. During this period among Rotatoria the genera Syncheata, Polyarthera, Philodina, Notholca, Collotheca, Asplanchna and within the Cladocera order, genus Bosmina and Daphnia accounted for the greatest share of population.The mean seasonal density of those orders was 6311.83± 381.22 and 5411.66±513.22 individual per m3 respectively. There was statistical significant difference in Zooplankton abundance among seasons (P< 0.05). In table 2 order of zooplankton of different genus in the lake behind the Dam has been identified separately.
Table 2. Different zooplankton genus known in Lar reservoire in the Years 2008 and 2009
| Genus of Zooplankton | orders of Zooplankton |
| Asplanchna brightwelli (Gosse), Polyarthera dolicopthera (Idelson), Syncheata pectinata (Ehrenberg), Notholca acuminate (Ehrenberg), Ascomorpha sp., Keratella cochlearis (Gosse), Collotheca sp., Philodina sp., Arachnida sp., Pedalia sp. | Rotatoria |
| Daphnia longspina (Sars), Daphnia pulex (De Geer), Bosmina coregoni (Baird), B. longirostris (Leydis), Macrothrix sp., Cladocera emberyoni | Cladocera |
| Cyclops vulgaris (Muller), C. smirnovi (Rylov), Eucyclops sp., Arctodiaptomus acutilobatus (Sars), Naupli copepoda | Copepoda |
Is noteworthy that in measuring physical and chemical factors, estimating of existing minerals in water has been done in the table 3.
Table 3: Average number of some physical and chemical factors studied in the research stations of Lar reservoir.
| Sampling Statins
Measuring Factors |
Dam crest | Middle of the Lake | Emmam pahnak | Ab-Sefid | Gezel Darah
|
| Weather temperature (0C) | 15.25 | 15 | 15.28 | 14.2 | 13.6 |
| Water temperature (0C) | 15.4 | 16 | 12.3 | 16.2 | 14.8 |
| Maximum sampling depth (m) | 42 | 28 | 20 | 15 | 6 |
| Amount of solved oxygen (ppm) | 8.3 | 8.11 | 8.13 | 7.86 | 7.25 |
| Water transparency (cm) | 33.33 | 27.3 | 22.8 | 17.8 | 21.5 |
| Ph of the water | 8.25 | 8.40 | 8.21 | 8.20 | 7.97 |
| Electrical conducting (µs/cm) | 266.2 | 253.4 | 282.6 | 257.2 | 238.5 |
| Solved Phosphate (ppm) | 0.022 | 0.028 | 0.018 | 0.027 | 0.018 |
| Nitrogen nitrate (ppm) | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Total hardness (ppm) | 147.75 | 141.25 | 139.75 | 144.5 | 148.5 |
| Solved Calcium (ppm) | 45.1 | 44.26 | 42.57 | 44.25 | 38.2 |
| Solved Bicarbonate (ppm) | 140 | 141.6 | 173.3 | 139.3 | 135 |
| Solved Silicat (ppm) | 4.76 | 3.53 | 4.31 | 4.32 | 2.92 |
| Solved Nitrogen ammonium (ppm) | 0.310 | 0.345 | 0.370 | 0.345 | 0.321 |
Discussion
Based on investigations made on dispersion and distribution of zooplanktons in various sites in the “Lar reservoir”, it can be divided in two parts: the deep part (with more than 40 m average depth) and shallow area (with average 5m) (Elmi, 2003). In the deep area, the zooplankton population tends to increase in early summer with the development of Cladocera and Rotatoria, which are of great diversity and thus prevent the domination in number of other orders.Changes in temperature might act as a trigger of seasonal processes, influencing the zooplankton directly (e.g. metabolic rates) or indirectly (e.g. modifications of the phytoplankton structure). In temperate lakes there is a noticeable decrease on zooplankton density in winter and increase and appearance of new species in other periods26. Syncheata constitute the dominant zooplanktonic population in late spring and early summer in Lar reservoir. The abundance and concentration of zooplankton, particularly Cladocera depend on the limnologic condition and eutrophic level of the lake10. So the abundance of the zooplankton has a positive relationship with the increase in eutrophication and water temperature and is in accordance with the results of our study. The zooplankton abundance is subject to a number of factors including water temperature, dissolved oxygen, organic and mineral substances and phytoplankton abundance, such a situation can fairly confirmed during summer when the large number of zooplankton is associated with increase of water temperature and organic load6.Williams32 held that Brachionus Sp., Keratella Sp., Polyarthera Sp. are the indexes of eutrophication status of water. However, Syncheata Sp. Reemerge in mid winter to take the bulk of zooplankton population. Researchers17 concluded that the increase of rainfall in summer is associated with the population increase of Rotifera. While Hutchinson9 mentioned that the availability of elements such as phosphate, nitrogen, iron & chloride in slow current water with a temperature of 18-25 0C and with a suitable organic load could significantly faster the development of Rotifera population. The production of phytoplankton in turn significantly contributes to the abundance of zooplanktons.The results obtained from a number of researches indicated that Cladocera and Rotifera constitute the major planktonic population and account for the bulk of zooplankton items observed as the food items of the fish caught in the “Lar reservoir “25. Cladocerans have been reported to generally increase in late spring and summer3, with a possible interrelationship between rotifers and cladocerans as predators and competitors8.The water temperature pattern of “ Lar reservoir “ is subject to environmental condition of the area in a way that the mean air temperature in spring is 16 0C temperature and reaches its maximum level of 25.5 0C during summer. The mean air temperature in autumn is 10 0C and plunges to below zero degrees in winter, resulting in severe frost & icing of “the lake”. Thus the variations in zooplankton abundance in water column in different seasons of the year is controlled by air temperature and the intensity of wind. In their investigation of zooplankton population in “Maku reservoir “Sabkara & Makarami (1997-2000) referred to the increase of crustacean population which was caused by suitable temperature occurred during May – June, whereas the during this period, the increased of Copepoda population was associated with a sharp decline in the number of Rotatoria.The Zooplankton studies carried out in the Maku reservoir (in northwestern of Iran) by Sabkara and Makarami22 showed that the zooplankton population of the area was composed of three branches such as Arthropod, Protista and Rotatoria out of which within Rotatoria the genus: Syncheata has the highest density22 .The expansion of Cladocera, particularly Daphnia in July can be attributed to temperature increase which ultimately led to the alteration of Daphnia reproduction mechanism. The temperature change created unsuitable living conditions for Cladocera & restricted their food sources. Such a rough living condition makes certain complications for the female eggs residing in the oviduct. Thus the reproduction system of Cladocera changes from parthenogenthesis to sexual reproduction. Meanwhile Valiulahi31 suggested that the possible consumption of female Daphnia by fish might have been the reason behind their shrinking population size. The zooplankton population tends to decrease as the water temperature declines in autumn. However the scale of the variations is not as wide as that of summer13.
The maximum monthly production of zooplankton occurs in the warm season of the year and the minimum production happens during the relatively cold spring that is the first month of the season. As the temperature drops in the autumn the monthly primary production is diminished by one gram per cubic meter, which can be accounted for the extremely abundant biomass developed in this period compared to that of early spring14.The slight Physico-Chemical difference among the five sampling stations located at difference parts of the “Lar reservoir have some bearings on the natural seasonal distribution of zooplankton. Light is one of the most effective factors on the vertical migration & distribution of zooplankton. The temperature increase is another positive factor that faster the development of zooplankton population11.Zooplanktons are of greatest fisheries significance during May-June. The production dynamics and quality of zooplankton in the reservoirs are suitable for consumption by young fish. The maximum population increase of minute Zooplanktons (i.e Rotifera and young crustaceans) coincides with the highest number of larvae that feed on them14.During May-June Rotifers & Cladocera rank first & second in terms of population size as the result of increased water and air temperature29. The reservoir water is fairly transparent in such a way that sunlight penetrates right down to the lake bottom which may be the reason why there is abundant zooplankton region. The vertical migration is the most common phenomenon amongst in zooplankton and is observable both in marine and fresh water resources based on their special behaviors. The light intensity variation is assumed to be the main reason triggering migration whereas the ultimate cause of such a behavior still remains a moot point19. The diurnal vertical movement pattern often functions separately and is influenced by factors such as heat (temperature), eutrophic environment, oligotrophic environment during daylight, and the energy saving rate. Most of the attempts at resolving these conflicts are based on either of the following two items:
The preparation for vertical movement of a reaction or statistical processing.
Avoidance from water during day time with a view to alleviate mortality caused by predation 15.
Most studies of the distribution of zooplankton were taken at depths of 10-0 m which in Mamadov14, also expressed this issue that in the case of the distribution of zooplankton from the shore toward the center of the reservoir water, maximum density of zooplankton was in the middle part of the 5 and 10 meters deep, which is showing that bye increasing deep into high, zooplankton density decreases, which can be due to light and water temperature.
Results of above studies also indicated the lake has the zooplankton factors used for drinking water, also in Akbulut1 expressed result of a one-year review that zooplankton spreading is (especially Rotatoria) highly dependent on physical and chemical changes of water, in his review he also knew the Rotifer the dominant zooplankton during cold season and the radiating antenna, the dominant group of hot season, as well as the two dominant images related to zooplankton approved1. It would be considered the size and the physical and ecological location of the lake behind the dam is the most affecting factors on dominance and density of zooplankton, therefore due to the small size and location of physical and ecological position, 97 percent of the zooplankton fauna in the Xiangxi Dam in China is known from Rotifer. The research also showed that if the physical and chemical conditions are optimized, Rutifer in surface, Cladocer in average depth and Copepod are dominance in deep level27. In connection with the effect of increasing elevation from the sea level during the 18 years of study, Larson et al in 2009 during a study on 103 Mountain Lakes in International Parks of United States, concluded that the average number of species of zooplankton reduced With height increasing and by increasing the depth the number of species increased significantly, especially rotifer12. Lar Dam Lake with a height of about 3400 meters from the surface of the sea level had 21 species of zooplankton that during the high-water months of spring zooplankton densities were higher.
Acknowledgement
I would like to present my deep appreciation to Mr. Dr.Azari Takami, the executive project manager, Iran’s environmental protection organization Mr. Dr. Saddogh, Polour environment guards, Mr. Dr. Rajabinejad and all colleagues in plankton laboratory in the research centre, particularly Eng. Mr. Sabkarah, Mrs. Makarami, Eng. Mrs. Khatib , Eng. Mrs. Madadi for the collaborations in the provision of samples.
References
- Akbulut, N., Akbulut, A., Park, Y-S. Relationship between zooplankton (Rotifera) distribution and physico-chemical variables in Uluabat Lake (Turkey). Fresenius Environmental Bulletin [Fresenius Environ. Bull.]. 17: 198- 200 (2008).
- Balayut, E.A. Stocking and introduction of fish in lakes and reservoirs in the ASEAN countries. FAO technical paper No.236.FAO,Rome, pp.82 (1983).
- Dokulil, M., Herzig, A. and Jagsch, A. Trophic relationships in the pelagic zone of Mondsee , Austria. Hydrobiologia, 191: 199-212 (1990).
- Edmonson,W.T. Fresh water biology. New York, London.John Wiley and Sons Inc.1248 P (1959).
- Elmi,A.M. The study on comprehension development plan of Laar National Park-Hydrobiology (Limnology) Environment preservation organization. Tehran.53 pp (2003).
- Fallahi,M. Hydrology & Hydrobiology of Anzali Lagoon project report. Gilan Fisheries Research Center .pp. 121 (1999).
- Gaacla, P. R., Nandini, S., Sarma, S.S.S., Valderrama, E. R., Cuesta, I. and Hurtado, M.D. Seasonal variations of Zooplankton abundance in the freshwater reservoir Valle de Bravo (Mexico). Hydrobiologia, 467: 99-108 (2002).
- Herzig, A. The analysis of planktonic rotifer populations: A plea for long-term investigations. Hydrobiologia, 147: 163-180 (1987).
- Hutchinson, E.A. A study of planktonic Rotifer of river Ganard, Essex,Ontario,M.S.C. Thesis University of Windsor, Ontario,Canada, pp. 58-86 (1970).
- Jappesen,E., Jensen,J.P. and Sondergaard,M. Response of Phytoplankton,Zooplankton and Fish to re-oligotrophication an 11-year study of 23 Danish Lakes. Aquatic Ecosystems Health and Management. 5:31-43 (2002).
- Kadri,A. Diatoms (Bacillariophyta) in the Phytoplankton of Keban Reservoir and their seasonal variations.Tr.J. of Botany . TURKEY. 42: 25-33 (1998).
- Larson, Gary L; Hoffman, R., McIntire, D., Lienkaemper, G., Samora, B. Zooplankton assemblages in montane lakes and ponds of Mount Rainier National Park, Washington State, USA. Journal of Plankton Research [J. Plankton Res.]. Vol. 31, no. 3, pp. 273-285 (2009).
- Mahdizadaeh ,Gh. A survey of the Zooplankton distribution and frequency in Gilan warm water fish production farms (Lakan district) in marine science and technology Journal,Vol 5 iss:3,4 .pp 85-77 (2006).
- Mahammadov, R. A. The Nakhjavan reservoir zooplanktons minsk publication, Academi of Science of Azerbaijan Republic , Baku in 38 pp (1990).
- McLaren,I.A. Effects of temperature on Growth of Zooplankton, and the adaptive value of vertical migration.J.Fish.Res.Bd.Canada , 685: 20-35 (1963).
- Newell,G.E. and Newell,K.C. Marin plankton, Hutchinson and Co.,London. U.K. 242 pp (1977).
- Oltra,R. and Miracle, M.R. Seasonal succession of Zooplankton population in the hypertrophic lagoon albufera of Valencia (Spain).Arch.Hydrobiel, 124: 187-204 (1992).
- Pontin,R.M. A key to the fresh water planktonic and semiplanktonic rotifera of the British Isles.Titus Wilson and Son.Ltd.178 pp (1978).
- Russel,G.E. Production of resistant varieties in vegetatively propagated crops in plant Breeding for pest and disease resistance. Printed in England by Billing and Sons Ltd.Guild for London,pp. 40 (1978).
- Ruttner-Kolisko, A. Plankton rotifers, biology and taxonomy, Austrian Academy of Science.147 pp (1974).
- Sabkara, J. and Makarami, M. The distribution & frequency of planktons & their role in Anzali Lagoon. Fisheries Research Scientific Journal iss No: 2, pp. 29 (2003).
- Sabkara J. and Makarami.M. A review on the plank tonic density and distribution in Maku Lake . Journal of fisheries sciences: IRAN.ISS 2, 12: 29-46 (2002).
- Safahi,S. Final report on comprehensive fisheries survey of Aras reservoir. Guilan Fisheries Research Center. Pp. 201 (1996).
- Salavatian,M., Fallahi, M. A study on the effects of varying Calcium concentrations on the growth rate and green algae biomass; Chlorella vulgareis. Journal of fisheries sciences. 14 :79-86 (2005).
- Salavatian,S.M., Abbasi ranjbar.K., Rajabinejad.R., Sayadrahim.M., Rajabi.T., Salavatian.S.H. and Emmampour Khoshdel.F. Investigation of Brown trout (Salmo trutta fario) food diet in rivers of Lar dam lake in the Fall. The first national cold water fishes conference. Iranian Fisheries research center, 12-14 May 2009, Tonekabon-IRAN. 276 P (2009).
- Sartori, L. P., Nogueira, M.G., Henry, R. and Moretto, E.M. Zooplankton fluctuations in Jurumirim Reservoir (Sao Paulo, Brazil): a three-year study . Braz. J. Biol., 69: 1-18 (2009).
- Shuchan.Z., Tang, T., Naicheng. F., Xiaocheng; C., Qinghua. Impacts of a Small Dam on Riverine Zooplankton. International Review of Hydrobiology [Int. Rev. Hydrobiol.]. Vol. 93, no. 3, pp. 297-311 (2008).
- Standard Method for examination of water and wastewater. American Public Health Association.U.S.A. 1194 P (1989).
- Sze, P. A biology of the algae. W.M.C. Brown publishers. 251 P (1986).
- Tiffany,L.H. and Britton, M.E. The algae of Illinois. Hanfer publishing company,New York,USA.407 P (1971).
- Valiulahi,G. Carp propagation and culture. Planning & programming Dept of Iranian Fisheries Organization .pp 72-78 (2002).
- Williams,L.G. Dominant Planktonic Rotifers of major water of the united states.Limnoloceanogr, 11: 83-91 (1966).

This work is licensed under a Creative Commons Attribution 4.0 International License.





