Manuscript accepted on : 15 June 2012
Published online on: --
Study into Oxidative Changes of Butter with Protective Coat
Pavel Mokrejs1, Jan Fenyk1*, Dagmar Janacova2, Petr Svoboda2* and Roman Cermak1
¹Department of Polymer Engineering, Faculty of Technology, Tomas Bata University in Zlin, Nam. T. G. Masaryka 275, 762 72, Zlin, The Czech Republic.
²Department of Processing Control and Applied Computer Science, Faculty of Applied Informatics, Tomas Bata University in Zlin, Nad Stranemi 4511, 760 05 Zlin, The Czech Republic.
Corresponding Author E-mail: mokrejs@ft.utb.cz
ABSTRACT: The contribution deals with employing starch-protein hydrolysate of amaranth flour to produce protective edible coatings on butter. Tests were run on 3 types of coating solutions. The first was made in such manner that to hydrolysate of 40-% dry matter content, 30-% (w/w) plasticiser (glycerol) was added and also 3-% (w/w) emulsifying agent (lecithin). The second and third contained an extra 2-% (w/w) antioxidant (ascorbic acid) and the third an extra 2-% (w/w) cross-linking agent (dialdehyde starch). Protective layers on butter were produced by coating with coat solutions. Butters were stored for a total of 96 days in refrigerator at a temperature of 7±1.5 oC and relative humidity of 36±3 %. The objective was to investigate the oxidation course of butter with protective coatings, butter wrapped in original wrapping and butter without wrapping/coat. Determined values were peroxide value, acid value and anisidine value. Results of butters with protective and edible polymer coatings displayed a similar oxidation course to that of butter in standard wrapping. Butter with a coat containing antioxidant exhibited slightly lower levels of peroxide value during storage than butter in original wrapping, which confirms good barrier properties to oxygen of the coating thus prepared.
KEYWORDS: Acid Value; Anisidine Value; Butter; Coating; Peroxide Value; Starch-Protein Hydrolysate
Download this article as:| Copy the following to cite this article: Mokrejs P, Fenyk J, Janacova D, Svoboda P, Cermak R. Study into Oxidative Changes of Butter with Protective Coat. Biosci Biotech Res Asia 2012;9(1) |
| Copy the following to cite this URL: Mokrejs P, Fenyk J, Janacova D, Svoboda P, Cermak R. Study into Oxidative Changes of Butter with Protective Coat. Biosci Biotech Res Asia 2012;9(1). Available from: https://www.biotech-asia.org/?p=9538 |
Introduction
Fats represent one of the basic components of food for man, containing vitamins A, D, E, K. Fats and oils have to be stored and transported in such manner that contact with solar radiation is avoided, as well as excessive contact with atmospheric oxygen. The U.S. Food and Drug Administration, U.S. Department of Agriculture and analogous national regulations define conditions for their storage. For vegetable fats or oils, recommended temperature is up to 20 oC, for fats or oils of animal origin it should not exceed 15 oC, and hardened fats may be stored at a temperature up to 20 oC. In case these conditions are not met, rancidity (oxidation) of fats appears and thus also their depreciation. Rancidity is a process where double bonds in unsaturated fatty acids contained chiefly in fats and other lipids get oxidised by atmospheric oxygen. It results in undesirable products, aldehydes and ketones in particular, which negatively alter action on health as well as the taste and smell of foodstuffs containing unsaturated fatty acids. The outcome is partial or complete depreciation of a foodstuff1,2. An example illustrating oxidation of double bonds in unsaturated fatty acids:
R1–CH=CH– R2 + O2 → R1–CHO–CHO–R2
In order to prevent these phenomena, a suitable wrapper has to be selected. It is well-known that mainly fats and butters in particular take over from surroundings smells that negatively affect their taste. Wrappings (chiefly for butter) should be sufficiently resistant to fat, should offer sufficient protection against oxygen (deceleration of oxidation) and also to water vapours effecting product dehydrogenation3,4. In foodstuffs wrapping technology there is an ever greater application of materials that are biodegradable and edible. We may also define coatings as very thin layers of material applied to the surface of a foodstuff in coherent layer preventing direct contact of the food with its environment. They limit permeation of gases (O2 and CO2), humidity and aromatic substances, prevent penetration of micro-organisms into food, decelerate oxidation of a meal but also improve appearance of a product and its general firmness and integrity. They can also be used as carriers for various foodstuff additives (for example, antioxidants, antimicrobial agents, flavour additives)5-7.
Edible wrappings are mostly based on proteins and saccharides. Animal proteins most used are gelatine, proteins of milk (casein and whey), caseinates or egg white. Vegetable proteins most frequently used for preparing coatings and films are wheat gluten, maize zein or soya protein, which also proved efficient when producing foils by extrusion8. Film flexibility gets improved with added plasticiser (for example, glycerol, sorbitol, polyethylene glycol). Added saccharides (starch, starch derivatives, cellulose derivatives, chitosan, carrageenan,) improve mechanical properties; cross-linking agents are employed to the same purpose. Added lipides and waxes allow achieving high gloss of film or coat and improved barrier properties. Protective coats are applied to cooled and frozen meats, poultry, pastry, cheese, fruit, vegetables, nuts, sweets, cereals, in manufacture of low-fat potato chips and croquets, and also in thermally processed products (cooked meat, frankfurters, etc.). Water-soluble bags for dried foodstuffs are also being produced9-15.
Objective of our contribution
Observing the oxidation course of butters provided with edible polymer coatings from starch-protein hydrolysate of amaranth flour during cold storage temperature, and its comparison with oxidation course of butter without wrapping/coat and of butter kept in original wrapping.
Materials and Methods
Amaranth flour was supplied by AMR Amaranth Co. (Hradec Kralove, Czech Republic). Dialdehyde starch (CAS No. 9047-50-1), glycerol (CAS No. 56-81-5), lecithin (CAS No. 8002-43-5) and ascorbic acid (CAS No. 50-81-7) were supplied by Sigma-Aldrich (St. Louis, USA).
Fresh butters
of standard weight 250 g and same lot, containing 82 % fat and no salt, were purchased in a retail chain. Quarters of butter were unwrapped and cut into samples measuring approximately 3.5×2.0x1.0 cm. Samples thus prepared were placed side by side about 1 cm apart on metal trays. A part of the samples was intended for coating, a part was without coat and 3 quarters of butter were kept in original wrapping.
Preparation of coating solutions
Amaranth flour, with use of commercially available enzymes BAN 480 L (α-amylase), AMG 300 L (glucoamylase) and CELLUCLAST 1.5 L (cellulase) supplied by Novozymes Co. (Denmark), was employed to produce starch-protein hydrolysate; preparation procedure is mentioned in our previous publications16-18. The given enzyme breakdown brought about 83-% conversion of starch and 32-% conversion of proteins. Starch protein hydrolysate was thickened on vacuum evaporator (temperature not exceeding 80 oC) to a 40-% (w/v) dry matter content. A total of 3 coating solutions were prepared. The first coating solution contained a 30-% addition (related to hydrolysate dry matter, w/w) of plasticiser (glycerol) and 3-% (w/w) emulsifying agent (lecithin); this solution is designated “Coating 1”. Glycerol and lecithin were added to thickened hydrolysate solution, and under permanent stirring at 70 oC the solution pH was adapted to 10.0±0.1 with added (approx. 2.5 mL) 5M NaOH; stirring continued until lecithin completely dissolved. Addition of plasticiser is necessary to ensure favourable mechanical properties of the coating (to prevent its breaking). The second coating solution contained an extra 2-% addition (w/w) of antioxidant (ascorbic acid); this solution is designated “Coating 2”. Ascorbic acid was added to the coating solution (30 oC warm) and was dissolved under stirring (3 minutes). The third coating solution contained an extra 2-% addition (w/w) of cross-linking agent (dialdehyde starch); this solution is designated “Coating 3”. Dialdehyde starch was added, the same as in the first case, together with glycerol and lecithin, to thickened solution of hydrolysate; ascorbic acid was added subsequently.
Formation of protective coatings and investigating oxidation of butter in storage
Quantitative investigation of butter oxidation was performed by determining peroxide value (PV), acid value (AcV), and anisidine value, (AnV)19-21. Coatings on butter were produced with a brush; the coating solution was tempered to room temperature (23±1 oC). Two coats were made, with a time interval of 15 min in between. Thickness of the coating on butter samples was 0.28±0.08 mm. Coated butters together with butters in original wrapping and butters without wrapping were then placed in refrigerator and stored at 7±1.5 oC and relative humidity 36±3 %. The refrigerator was opened 6 times a day for 5-minute periods to simulate conditions of current refrigerator daily usage. Determinations performed on the butters after 7, 14, 28, 42, 56, 70, 84 and 98 days were peroxide value, acid value and anisidine value. Each test was run on three samples and calculation gave the arithmetic mean; standard deviation was ±5 %.
Results and Discussion
Dependence of peroxide value (PV) on duration of storage is indicated in Fig. 1. From the course of peroxide value curves it is obvious that during approximately first 45 days since start of test, differences between the peroxide values of investigated butters are not too prominent. The following gradual and greatest increase in PV of butter without coat is visible. Butter kept in original wrapping exhibits an increase in PV during storage that is slow and PV levels are second lowest. Increased PV of butters provided with three protective coats was in all cases lower than increased PV of butter samples without coating. Growth in PV is particularly obvious with the butter sample treated with Coating 2, and is slightly lower than that of butter kept in original wrapping. The difference between lowest PV levels (butter in original wrapping and also butter with Coating 2) and highest PV levels (butter without wrapping) after approx. 56 hours of storage is almost twofold.
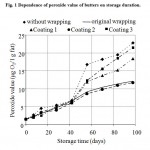 |
Figure 1: Dependence of peroxide value of butters on storage duration.
|
The dependence of acid value (AcV) on storage duration is indicated in Fig. 2. Development of this value from start of test displays an obvious difference between AcV of butters without wrapping and butters in original wrapping. During the first approx. 42 days of storage, AcV of butters treated with three tested coatings ranges between minimum AcV level (for butters in original wrapping) and maximum AcV level (for butters without wrapping). After more than 42 days of storage, AcV level of butters with Coating 2 and Coating 3 are slightly higher than that of butter kept in original wrapper, nevertheless, always lower than of butter without wrapping. Contrarilly, with the butter samples treated with Coating 1 AcV levels after more than 45 days of storage are even slightly lower than that of butter kept in original wrapping.
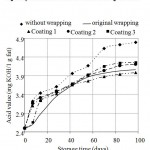 |
Figure 2: Dependence of acid value of butters on storage duration.
|
The dependence of anisidine value (AnV) of butters on storage duration is indicated in Fig. 3. After 7 days of storage, difference between development tendency of AnV of butters without wrapping and butters in original wrapping is quite obvious. Lowest increase in AnV during whole storage duration was recorded with butter kept in original wrapping. After 42 days of storage, AnV level of butters without coat prominently grows, on the opposite, slow growth was recorded with butters in original wrapper and with those bearing Coating 3. After 70 days of storage, AnV level of butters with Coating 3 is almost identical with that of butter in original wrapper and is about 1.35 times lower than the AnV level of butters without wrapper.
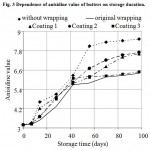 |
Figure 3: Dependence of anisidine value of butters on storage duration.
|
Conclusion
Protective coatings on butter were produced with a starch-protein hydrolysate of amaranth flour of 40-% (w/v) concentration. Three coating solutions were prepared. Each contained a 30-% (w/w, per hydrolysate dry matter) addition of plasticiser (glycerol) and 3-% (w/w) addition of emulsifying agent (lecithin). The second and third solution contained an extra 2-% (w/w) addition of antioxidant (ascorbic acid) and third an extra 2-% (w/w) addition of cross-linking agent (dialdehyde starch). Samples of butter were stored at 7±1.5 oC and relative humidity 36±3 %. Tests confirmed that a protective and edible polymer coating applied on butter allows achieving during storage at cooling temperature a degree of oxidation similar to that of butter wrapped in standard wrapper. Butter provided with a coat containing added antioxidant recorded a slightly lower increase in peroxide value than butter in original wrapping, which proves good barrier properties against oxygen of coating produced in this manner.
Acknowledgements
This article was created with support of Operational Program Research and Development for Innovations co-funded by the European Regional Development Fund (ERDF) and national budget of the Czech Republic, within the framework of project Centre of Polymer Systems (reg. number: CZ.1.05/2.1.00/03.0111).
References
- Lawson, H.: Food Oils and Fats: Technology, Utilization, and Nutrition, 1st edn. New York: Chapman & Hall, 1995; pp 3-65.
- Bockisch, M.: Fats and Oils Handbook, 1st edn. Champaign: AOCS Press, 1998; pp 53-120.
- Brown, H., Williams, J.: Package product quality and shelf life. In: Food Packaging Technology (Coles R, McDowell D, Kirwan MJ, eds). Oxford: Blackwell Publishing, 2003; pp 65-94.
- Wilbey, R.A.: Butter. In: Dairy Fats and Related Products (Tamime AY, ed). Oxford: John Wiley & Sons, 2009; pp 86-107.
- Robertson, G. L.: Food Packaging: Principles and Practice, 1st edn. New York: Marcel Dekker, 2005; pp 338-380.
- Domb, A.J., Kost, J., Wiseman, D.M.: Handbook of Biodegradable Polymers, 1st edn. Boca Raton: CRC Press; 1997, pp 307-318.
- 7. Kaplan, D.J., Mayer, J.M., Ball, D., McMassie, J., Allen, A.L., Stenhouse, P.: Fundamentals of biodegradable polymers. In: Biodegradable Polymers and Packaging (Kaplan DL, Thomas EL, Ching CTK, eds). Lancaster: Technomic Publishing Company, 1993; pp 1-42.
- Smith R.: Biodegradable Polymers for Industrial Applications, 1st edn. Boca Raton: CRC Press, 2005; pp 4-21.
- Gennadios, A., Hanna, M.A., Kurth, L.B. Application of edible coating on meat, poultry and seafoods: A review. Lebensm.-Wiss. Technol., 1997; 30(4): 337-50.
- Cha, S.D., Chinnan, M.S. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr., 2004; 44(4): 223-37.
- Kayserilioglu, B.S., Stevels, W.M., Mulder, W.J., Akkas, N. Mechanical and biochemical characterisation of wheat gluten films as a function of pH and co-solvent. Starch, 2001; 53(8): 381-6.
- Aminlari, M., Ramezani, R., Khalili, M.H. Production of protein-coated low-fat potato chips. Food Sci. Technol. Int., 2005; 11(3): 177-81.
- Herold, T.J, Hachmeister, K.A., Huang, S., Bowers, J.R. Corn zein packaging materials for cooked turkey. J. Food Sci., 1996; 61(2): 415-8.
- Mate, J.I., Kruchta, J.M. Whey protein coating effect on the oxygen uptake or dry roasted peanuts. J. Food Sci., 1996; 61(6): 1202-7.
- Okamoto, S.. Factors affecting protein film formation. Cereal Foods World, 1978; 23(5): 256-62.
- Mokrejs, P., Langmaier, F., Janacova, D., Mladek, M., Kolomaznik, K. Thermal study and solubility tests of films based on amaranth flour starch-protein hydrolysate. J. Therm. Anal. Calorim., 2009; 98(1): 299-307.
- Mokrejs, P., Krejci, O., Hrncirik, J., Janacova, D. Decelerating weight loss of harvested strawberries by applying edible coatings based on starch-protein hydrolysate of amaranth flour. Orient. J. Chem., 2009; 25(4): 833-9.
- Mokrejs, P., Hrncirik, J., Janacova, D., Vasek, V., Cermak, R. Solubility of edible films from amaranth flour enzymatic hydrolysate modified with dialdehyde starch. Asian J. Chem., 2010; 22(3): 1982-92.
- BS EN ISO 3960:2010. Animal and vegetable fats and oils, Determination of peroxide value, Iodometric (visual) endpoint determination.
- ISO 660:2009. Animal and vegetable fats and oils, Determination of acid value and acidity.
- ISO 6885:2006. Animal and vegetable fats and oils, Determination of anisidine value.

This work is licensed under a Creative Commons Attribution 4.0 International License.





